
Distillation is a reversible physical separation process that utilizes the relative volatility of the mixture components. In fluid mixtures, the more volatile compound is easier to isolate because it escapes the solution as gas more quickly even before the solution boils.
The fundamentals of distillation are based on the physical chemistry of the mixtures to be separated. When it comes to distillation, the most important property to look into is vapor pressure.
Vapor pressure is an intrinsic property of a substance which only varies with temperature. A certain substance will have a higher vapor pressure at higher temperatures. This is because the increased kinetic energy of the molecules due to heat causes the substance to phase change to gas.
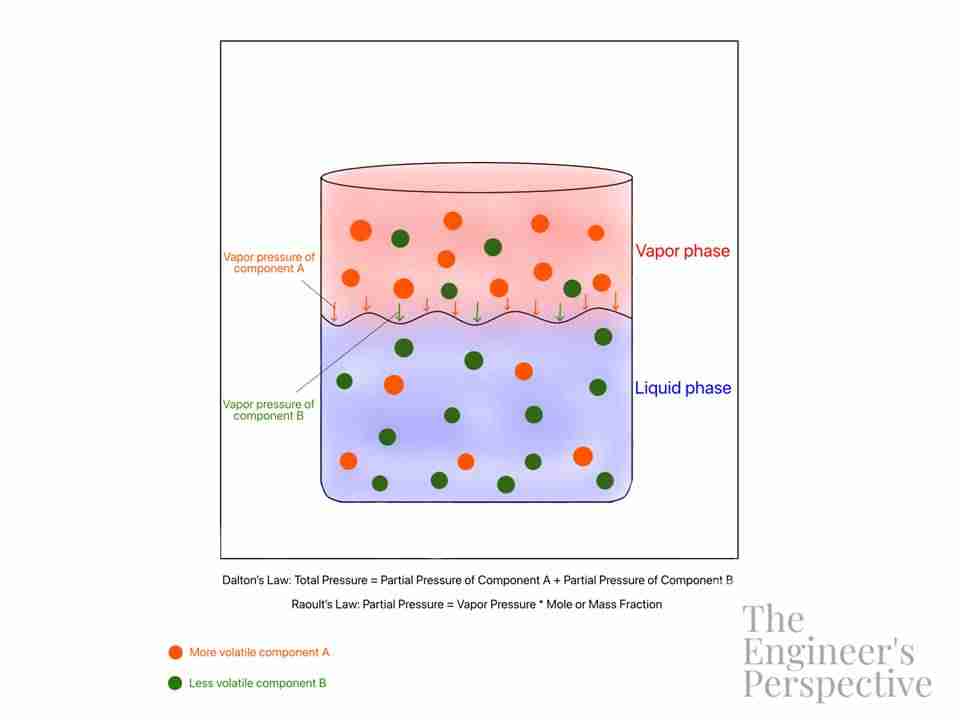
In a closed system, a solution at equilibrium has equal rates of evaporation and condensation taking place. Vapor pressure is the pressure exerted by the gas on the condensed phases (liquid or solid) at a given temperature.
There are 2 important laws that illustrate how vapor pressure works in mixtures — Raoult’s Law and Dalton’s Law.
The figure below shows an example of a process flow diagram for distillation.
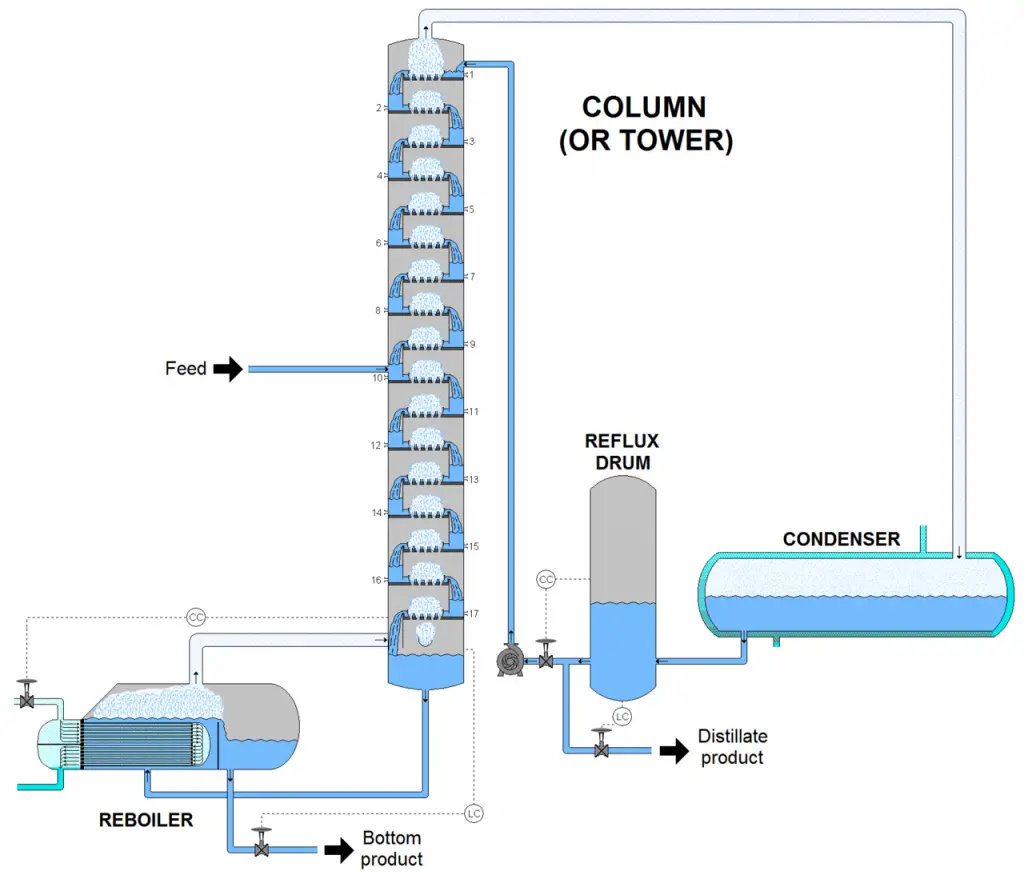
The feed mixture enters the column where distillation column internals (trays, plates, or packings) provide vapor-liquid contact. When the mixture is heated to its boiling point, vaporization starts — liquid changes to its gaseous phase.
The vapor-rich stream with the more volatile component rises and exits at the top of the column and enters the condenser for cooling — where condensation occurs. The condenser has a cooling fluid that turns the vapor back to its liquid phase. The condensed vapor accumulates in the reflux drum and exits as the distillate. Part of it is recycled back to the column as liquid reflux.
The liquid-rich stream exits at the bottom of the column and leaves the reboiler as the bottoms by-product. The reboiler provides heat to the liquid at the bottom, and when it vaporizes, some of it is sent back to the column.
Since different mixtures behave in different ways, there is no singular technique that applies the principles of distillation. There are several ways on how to achieve the purest distillate possible.
The vaporization-condensation cycle happens in a single theoretical stage at fixed temperature and pressure conditions.
Fractional distillation is used on mixtures whose components have a closer boiling point difference — less than 70°C. It occurs in multi-stage vaporization-condensation cycles to produce a distillate with a higher purity than simple distillation.
It is commonly applied in oil refineries and chemical plants. Through fractionation columns, crude oil gets separated into gasoline, diesel fuel, kerosene, and jet fuel fractions — distillates with similar boiling points, molecular weights and properties
Steam distillation is used when the mixture contains at least two immiscible liquids with temperature-sensitive components. It is most commonly applied in extracting essential oils from flowers and herbs. Since aromatic compounds are heat-sensitive, steam distillation preserves its quality.
It works by indirectly heating the mixture in a separate chamber with low-pressure steam. Vapor pressure increases with temperature, hence, it boils at lower temperatures. As the more volatile compound evaporates with the steam, it passes through a condenser where the distillate is collected. By gravity, the distillate separates to two layers — water and the immiscible component, usually an organic compound.
Vacuum distillation is used on mixtures with high boiling points. Rather than heating the mixture to bring it to its normal boiling point, it is more cost-effective when the system pressure is reduced. Consequently, the mixture vaporizes quickly at a lower temperature — saving time and energy.
It works by creating a vacuum through a vacuum pump or steam ejector so the system pressure brings down to below 1 atm. After the more volatile compound vaporizes, it works like simple distillation (if single-stage) or fractional distillation (if multi-stage) where the vapor is condensed and distillate is collected.
Azeotropic distillation is a specific technique that is used to separate azeotropic mixtures — two or more liquids with a fixed composition that distills as if it was a single compound with one constant boiling point. Azeotropes deviate from Raoult’s Law so applying simple or even fractional distillation is futile.
With azeotropic distillation, an inert volatile substance called an entrainer is added to the system to increase the relative volatility of the mixture. The entrainer breaks the original azeotropic feed mixture, and displaces either of the feed components to form a new azeotrope. The feed mixture with the entrainer then undergoes fractional distillation wherein the distillate contains the more volatile compound, and the bottoms contains the azeotrope formed with the entrainer.
For example, toluene and benzene are the common entrainers used to separate the azeotropic mixture between ethanol and water.
Cryogenic distillation is used on gas-gas mixtures whose components have boiling points at freezing temperatures. It is called as such because the process occurs at cryogenic temperatures ranging from -170°C to -190°C. Gases are liquefied at these temperatures which makes it easier to purify.
The most common example of cryogenic distillation is separating oxygen and nitrogen from air. Air is a homogenous mixture of gases, most of which have to be separated from oxygen and nitrogen since these are essential raw materials for industrial processes.

Among all distillation types, simple distillation and fractional distillation are the most widely used in the industry. The other types of distillation come in when additional separation techniques are added to the mixture to enhance the efficiency of the distillation process. However, they still follow the configuration of either fractional or simple distillation (more so with fractional if dealing with complex mixtures).
Batch distillation is used for low-volume production with highly specific concentrations such as in the production of spirits and in the pharmaceutical industry.
Distillation is best used on homogeneous binary mixtures where one component is more volatile than the other.
Whereas distillation separates mixtures in terms of vapor pressure difference, extraction is based on the solubilities of its components. Distillation also requires heating the mixture to boiling, but it is not necessary with extraction.
Aside from those types mentioned previously, there are also other special types like zone distillation, short path distillation, pressure-swing distillation, extractive distillation, and more.
The purified product is commonly referred to as the distillate which is concentrated with the more volatile component. Meanwhile, the bottoms by-product will also yield the non-volatile component.
Remember, you have the information, but more importantly, you have the Engineer’s Perspective!