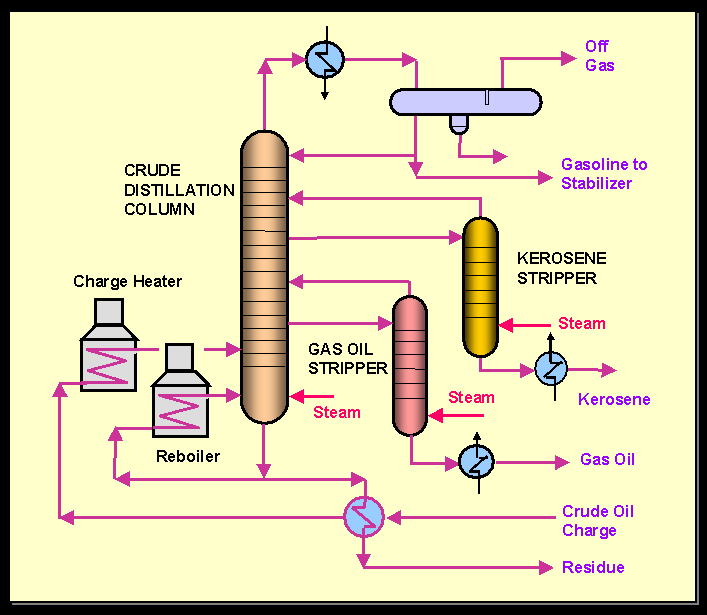
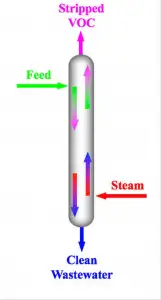
Steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to separate temperature-sensitive volatile components in a solution at a much lower temperature than its boiling point.
Vapor pressure and boiling points are the key drivers in any of the many types of distillation. Steam distillation is no different.
Steam distillation functions on the principle that steam (water) forms an immiscible, non-ideal mixture with the components to be separated. Each component in the mixture will, as a result, establish its own vapor pressure independent of its concentration as the temperature increases.
Steam, which typically has a lower boiling point than the volatile component in the mixture, will now account for part of the total equilibrium vapor pressure as the mixture boils. This effect lessens the thermal energy required for the mixture to reach atmospheric pressure.
Raoult’s law states that the vapor pressure exerted by a single component in an ideal mixture is lowered by the addition of solute.
In other words, the partial pressure of a single component in an ideal mixture is equal to its molar composition multiplied by the total equilibrium vapor pressure.
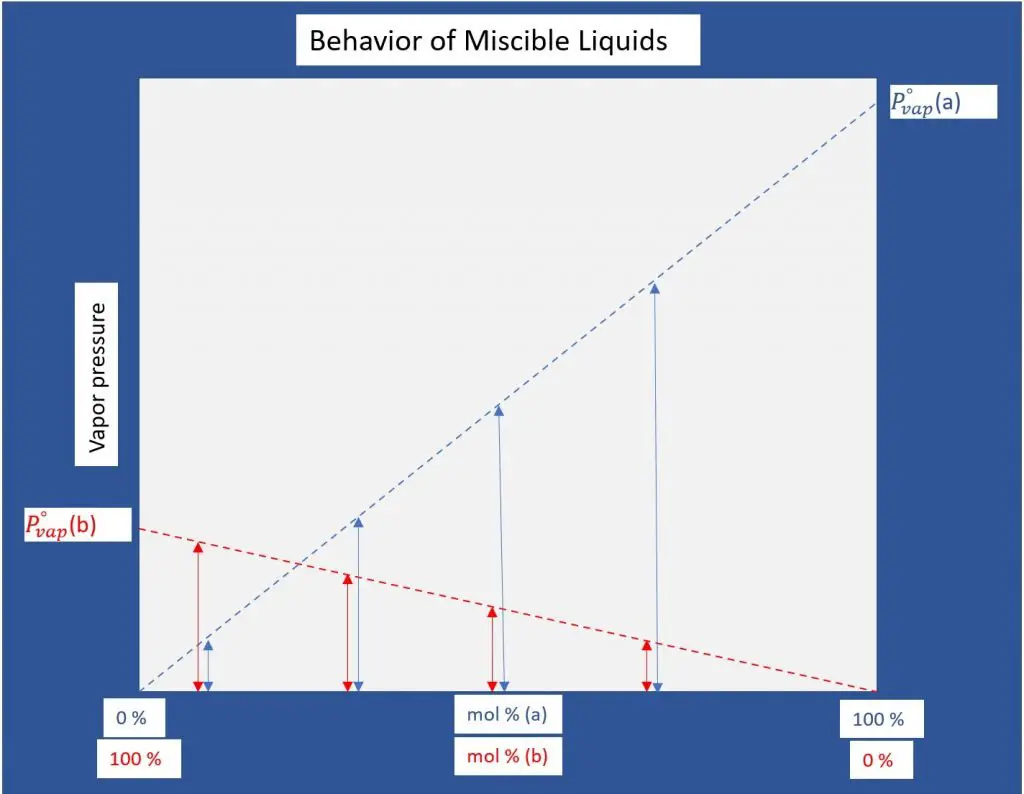
Dalton’s Law then dictates that the total equilibrium vapor pressure is a sum of the individual partial pressures of the components. This is the case only for ideal mixtures which are inherently miscible and therefore what distinguishes a simple distillation process from steam distillation.
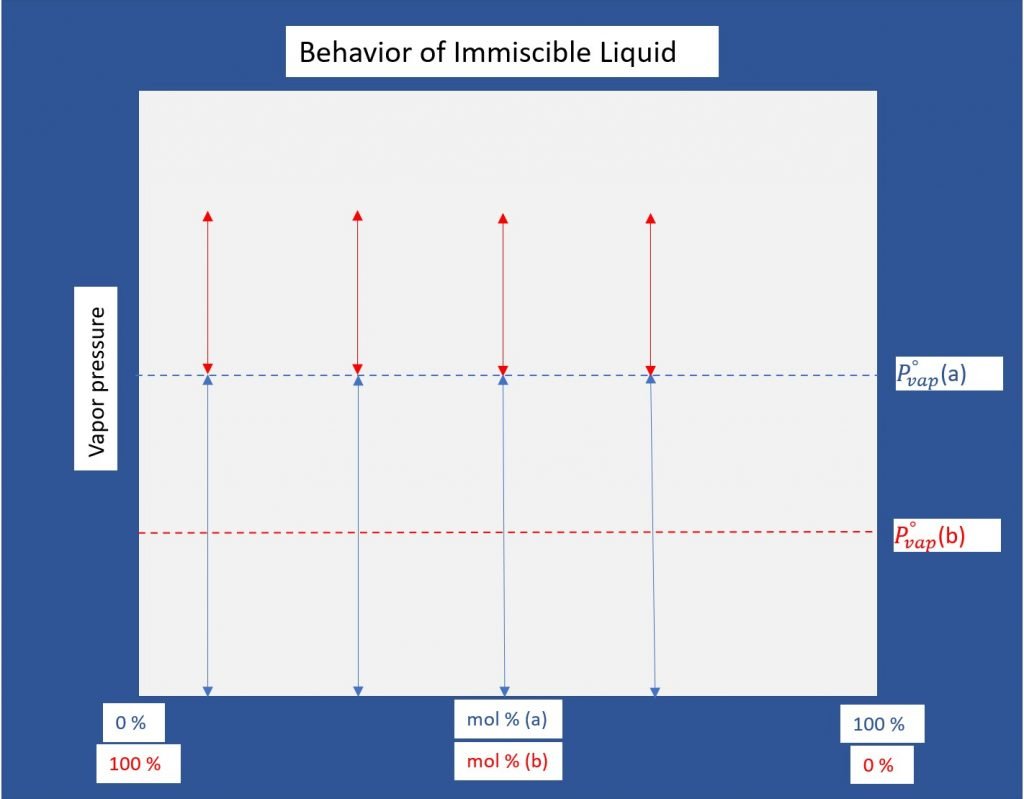
One important consideration in steam distillation is the need for constant agitation of the mixture. If left unagitated, the liquid components will form layers on top of each other resulting in a vapor pressure equal to that of the top layer [2].
Agitation is inherently achieved in a continuous process as the downcoming feed contacts the steam fed at the bottom of the column. In batch and lab scale processes, this is accomplished via an agitator or stirrer.
The first applications of distillation can be dated as far back as 3500 BC. Although, innovation of the steam distillation process is speculated to have been pioneered in the 16th century by French scientist Claude Dariot [1].
Steam distillation has also been credited to have Persian origins dating back to the 11th century. It’s creation and first applications have been attributed to Persian chemist, Ibn Sina (Avicenna) who used the process to extract essential oils from roses for aromatherapeutic “healing” purposes [4].
Some of the industrial applications of steam distillation include:
A common application of steam distillation is found in fragrance and essential oil extraction. Aromatic compounds like terpenes and esters are extracted from leaves, stems and flowers yielding essential oils and “flower waters” such as rose water, lavender water etc.
In the video below, Limonene, a terpene found in lemons, is extracted from its peel in a lab-scale steam distillation process:
Most terpenes and esters found in organic matter require high temperatures to separate from their non-volatile components. In the case of organic matter, the non-volatile constituents thermally degrade at these high temperatures due to the limited thermal stability of their bonds. This is the primary advantage of steam distillation.
Steam distillation also allows for ease of separation between the desired product and the condensed steam due to their immiscibility. The volatile condensate will typically be less dense than the condensed steam and can be separated through decantation.
The main drawback of steam distillation in this application is its time-sensitive nature. The process can typically last between 30 minutes to 6 hours depending on the plant and its quantity [5]. This is due to prevalence of steam in the vapor phase as the mixture is brought to a boil.
Steam distillation is also referred to as steam stripping in this application. Volatile organic compounds are stripped from their housing mixtures in either a continuous or batch process using superheated steam.