
Separation techniques are vital for isolating harmful or useful mixture components by leveraging an understanding of the mixture’s physical and chemical properties.
For example, the refined diesel or gas obtained from gasoline stations is refined from crude oil by separating the components through their difference in vapor pressure properties. Another example is the purification of process water that came from manufacturing plants. Wastewater treatment separates the hazardous substances through their differences in particle size and solubility properties.
Such is the reason why conventional separation techniques were engineered.
Unlike mixing, separating a mixture is thermodynamically unfavorable. Although not spontaneous, it is possible to reverse the process through heat or work, especially when there are no chemical reactions involved.
There is no universal way of separating mixtures because each mixture has its unique behavior. Typically, mixture components are separated by:
There are 2 types of mixtures — homogeneous and heterogeneous.
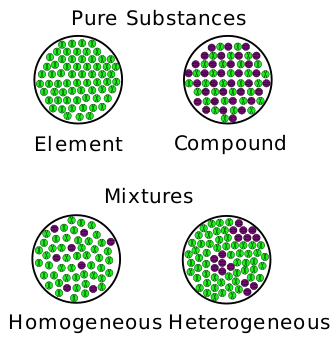
Homogenous mixtures (e.g. air) consist of substances that are evenly distributed throughout a single phase. Meanwhile, heterogenous mixtures (e.g. oil spill) do not have a uniform composition, forming more than one phase.
Separation techniques can be classified into two categories:
Here are the different types of separation techniques based on these categories:
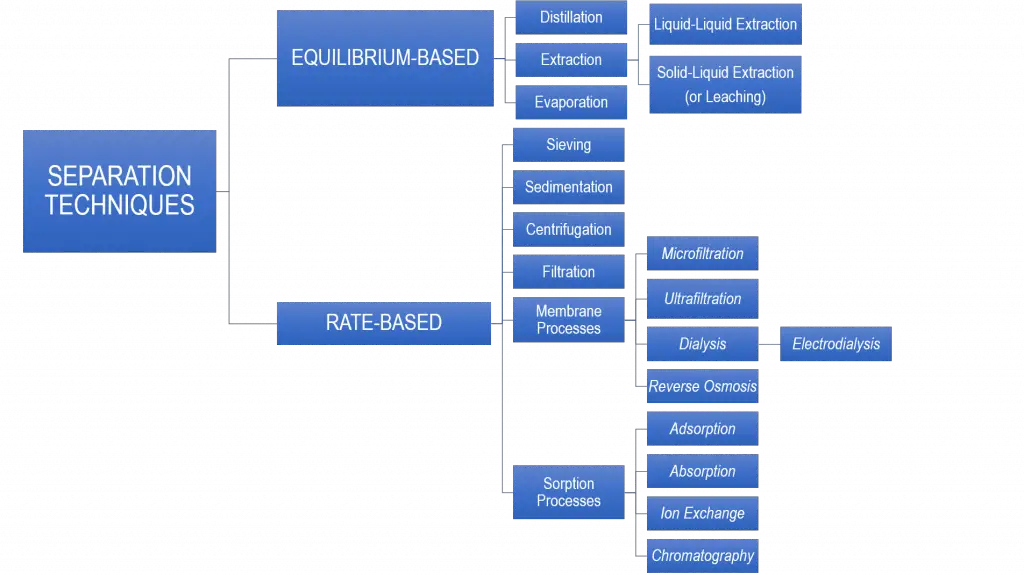
Each separation technique has a unique basis of separation, which lies on the differences of the physical or chemical properties between the mixture components.
Here is a table that summarizes the bases of separation for the different types of separation techniques:
SEPARATION TECHNIQUE | BASIS OF SEPARATION | ENGINEERING EQUIPMENT | COMMON INDUSTRIAL APPLICATION |
Distillation | Vapor pressure / Boiling point | Distillation columns and heat exchangers (reboilers, condensers) | Desalination, oil refining, chemical processing, air liquefaction, alcoholic beverages, pharmaceutical |
Extraction | Solubility | Mixer-settlers, extraction columns, centrifuge | Mining, metal processing, pharmaceutical, agriculture, food industry |
Evaporation | Vapor pressure / Boiling point | Single-effect and multiple-effect evaporators | Sugar processing, pulp & paper, food & beverage, plastics |
Sieving | Particle size | Sieve shakers | Pharmaceutical, food & beverage, construction |
Sedimentation | Density | Sedimentation basins, settling tanks, clarifiers | Wastewater treatment |
Centrifugation | Density | Centrifuge, cyclone separators | Food processing, chemical, pharmaceutical |
Filtration | Particle size | Filter press, belt filters, rotary vacuum filters | Wastewater and water treatment, pharmaceutical, food & beverage, air pollution control |
Membrane | Particle size | Membrane modules, RO systems, desalinators | Wastewater and water treatment, chemical, biotechnology, food processing, pharmaceutical |
Sorption | Sorbent-Sorbate Affinity | Absorption towers, adsorption towers (fixed bed or fluidized bed), HPLC columns, water softeners, deionization systems | Air pollution control, wastewater and water treatment, pharmaceutical, biochemical research, food & beverage |
Their main characteristic is when one or more phases of the mixture are brought in direct contact with each other until the system attains equilibrium — which is when the rates of the reversible phase or chemical changes are simultaneous and equal.
It involves a repeated cycle of vaporization-condensation until equilibrium is achieved. Components of homogeneous mixtures with significant vapor pressure differences are best separated through distillation.
There are different types of distillation (most commonly simple or fractional distillation) and they differ in application on how significant the vapor pressure differences are among the mixture components.
Extraction is commonly used in separating heterogeneous mixtures with immiscible phases. A third component, called the solvent, is added to the original mixture to isolate the solute. Due to differences in solubility, the solute transfers from the raffinate — the original mixture that has been removed of impurities — to the extract — the resulting mixture between the solvent and the solute.
There are 2 types of extraction namely:
The 3 components in an LLEx extraction system are the fresh solvent, the solvent, and the solute in the mixture. The fresh solvent and the solvent in the mixture must be immiscible with each other to form 2 phases — an aqueous phase and an organic phase. Take note that the solute must be more soluble with the fresh solvent than its original solvent for extraction to take place.
No reaction must occur to avoid more impurities. Although, there are cases of reactive extraction, which in this case, may need additional separation methods downstream.
Leaching separates solid mixtures by introducing a fresh solvent. One of the components in the solid mixture must be soluble with the fresh solvent for extraction to take place. The leaching process is just one of the many refining methods in the metal processing industry.
Evaporation concentrates a solute by drying out most of the solvent. Evaporation is quite similar to distillation in the sense that it separates a mixture through their difference in volatility. Although, their main difference is that in evaporation, the mixture separates without reaching its boiling point and condensation does not necessarily have to take place.
The following separation methods isolate mixture components through a medium, usually a barrier, by way of a driving force (differences in pressure, concentration, temperature, or electric field). Aside from thermodynamic equilibrium, transport properties must also be considered such as the permeability of the membrane or filter.
By gravitational force, sedimentation separates heterogeneous solid-liquid mixtures through their difference in densities. This occurs in settling tanks or clarifiers where the mixture separates into two layers — the heavier solid phase at the bottom, and the lighter liquid phase at the top.
Sedimentation tanks are designed to have high depths, usually 15 to 20 feet for conventional basins. The system must not be turbulent to allow settling, which usually takes 3 to 4 hours on average.
Just like sedimentation, centrifugation also works through the mixture components’ differences in densities. However, the process is accelerated via the use of a mechanical device called a centrifuge.
In a centrifuge, the rotating motion of the equipment creates a centrifugal force that pushes the heavier material towards the wall of the bowl. Hence, the mixture is separated into layers, wherein the lighter phase is nearer to the center of the bowl.
Sieving separates solid mixtures or liquid mixtures through their differences in particle size using a series of sieve trays.
In a sieving machine, multiple sieving plates are stacked together with sieving trays that vary in diameters of punctured holes. As the mixture is agitated through vibration, the particles are forced to filter through the screens. The finer particles will settle to the bottom tray while the coarser materials are found in the top sieve trays.
Filtration separates solid-liquid mixtures through their difference in particle size by trapping the solids using fabric filters.
Initially, the liquid that comes out of the filter press would contain a few solids. But as more of the solids deposit on the filters, forming what’s called a filter cake, pressure in the system increases. Hence, the flow reduces and a clearer liquid. After such time, the filter cake is scraped off the filters before separating another feed mixture.
It is a collective term for separation techniques that use a semi-permeable membrane that acts as a selective barrier. It follows the same principle as in the filtration method, but the process is more advanced because it uses finely porous membrane modules. The phase that didn’t pass through the membrane is called the retentate, while the solvent-rich phase stream is called the permeate.
There are several kinds of membrane processes and they differ in the driving force and the particle size retained.
Type of Membrane Process | Driving Force / Gradient | Separation size range |
Microfiltration | Pressure | 10 – 0.1 µm |
Ultrafiltration | Pressure | < 0.1 µm – 5 nm |
Reverse Osmosis | Pressure | < 5 nm |
Electrodialysis | Electric Field | < 5 nm |
Dialysis | Concentration | < 5 nm |
These processes pertain to the accumulation of one phase within another phase, or onto the phase boundary only due to the affinity between the sorbent and sorbate. Here’s a better way of distinguishing between the two — in a sponge filled with water, the sponge acts as a sorbent while the water serves as the sorbate in the sorption mechanism.
Among several sorption mechanisms, the most commonly used separation techniques in the industry are absorption and adsorption. On a much smaller scale, there is also chromatography and ion exchange.
It involves the mass transfer of particles across the bulk phase of another material. Gas absorption is the most common separation method in the industry, especially as air pollution control devices, for the removal of hazardous gases such as CO2, NOx, and SOx.
The equipment design is usually as packed columns where a liquid absorbent is sprayed into. The spent absorbent is then recycled through desorption in stripping columns.
It involves the adhesion of particles on the surface of a substance that accumulates only at the surface.
The most widely available adsorbent is activated charcoal. Aside from being cheap and inert, it also has high porosity with an average of 1,000 square meters of surface area per gram. Thus, it can adsorb solid or gas impurities from liquid mixtures.
Chromatography uses a porous sorbent as a stationary phase and a solvent that moves through the column as the mobile phase. Depending on their affinity to the stationary phase (usually resins), the components in the mobile phase move through the column at different rates and are collected respectively.
Chromatography carbon is based on adsorption mechanisms involving solute molecules being bonded directly to the surface of the stationary phase.
It involves a chemical reaction in which free mobile ions of a solid (the ion exchanger), are exchanged for different ions of a similar charge in solution. This process is common in water treatment plants, especially in water softening by removing unwanted ions like Ca2+ and Mg2+.
For homogenous fluid mixtures, the most common separation method is distillation. Meanwhile, it is filtration for heterogeneous mixtures.
The most efficient separation technique is arbitrary to the type of mixture being separated. There are several separation techniques to choose from, and each of them has conventional applications depending on the industry.
For equilibrium-based separation methods, the major factors are temperature and pressure as these are the process parameters that can be easily manipulated in industrial plants.
For rate-based separation methods, the membrane, filter, and sorbent all affect the rate and thus, efficiency of separation. Process parameters like temperature and pressure also play a role.