A packed distillation column is a cylindrical vessel filled with packing materials that enhance continuous mass transfer between two feed fluids (liquid-liquid or gas-liquid) by effectively increasing the contact surface area.
Typically, one of the fluids preferentially wets the packing, flowing as a film over its surface while the second fluid flows over the remaining volume of the vessel.
A packed distillation column can be filled with random individual packings (e.g. Pall Rings and Berl Saddles), can be structured, grid packed, or filled with catalysts or adsorbents such as zeolite or activated carbon.
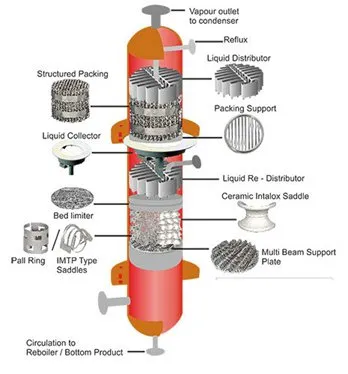
The main components of a packed column are:
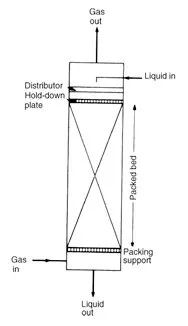
In gas/vapor – liquid systems, the liquid is the wetting fluid while the gas/vapor rises through the column making close contact with the downcoming liquid through the packings’ surface. In distillation set-ups such as in fractional distillation, the more volatile component of a mixture progressively transfers to the vapor phase while the less volatile component condenses out into the liquid. For liquid-liquid extraction, the solute is transferred to the solvent as the fluids come in contact with each other in the surface of the packings.
The following terms are commonly used in the operation of a packed column:
Pressure drop is the pressure difference between any two points along the packed column. It accounts for mechanical forces such as the gravitational and frictional forces. It essentially dictates the efficiency of mass transfer.
While there are various approaches to determining the pressure drop, the most accurate method is through the manufacturer’s own literature whenever the data is available.
Pressure drop inside a packed column is greatly affected by the packing factor — the packing’s surface area per unit volume (a) divided by the cube of the packing void fraction (ε):
Fp = a/ε3
which has a dimension of 1/ft or 1/m. However, packing factors nowadays do not correspond to a/ε3 anymore but are rather determined by the manufacturer so as to match a generalized pressure drop calculation. This is due to more efficient packing shapes that can have a higher surface area without causing too much pressure drop especially for structured packings.
The main difference between a packed column and a tray column is the mode by which the two fluids interact. Tray columns use a system of trays and weirs while packed columns use packing materials to promote fluid contact. Between the two, pressure drop is much lower in packed columns. To give an idea of the scale difference, the pressure drop across a structured packed column is only one-sixth of that across a tray column of the same height.
A thorough discussion between packed and tray columns is discussed here.
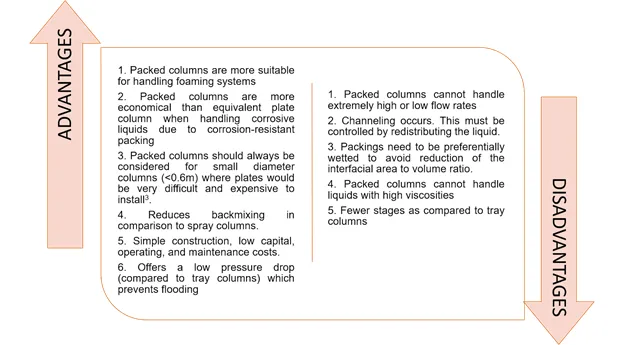
In summary, these are the advantages and disadvantages of packed columns:
1. What is the difference between capillary column and packed column?
While both packed columns and capillary columns are used in gas chromatography, packed columns are used more in distillation, gas absorption, and liquid-liquid extractions.
In packed columns, the stationary phase is situated in the cavity of the packed column. On the other hand, the stationary phase flows over the inner surface of the cavity of the capillary column.
2. Why is a packed column used in fractional distillation?
Packed columns are used in fractional distillation when access is limited (e.g. small diameter distillation columns) and if corrosion control/resistance is essential. Although, packings are still not recommended for large diameter columns or where a higher turn down ratio is required.
3. How do you find the height of a packed tower?
There are 2 common theoretical approaches — equilibrium stage analysis (through HETP) and mass transfer analysis (through HTU & NTU).
HETP is the height of the packing equivalent to a theoretical plate in which enrichment of the gas occurs. The theoretical number of plates is determined via the McCabe-Thiele Method. There are HETP values for every type of packing to be used in the column.
Meanwhile, the height of a packed tower can also be based on HTU (height of transfer units) x NTU (number of transfer units). The HTU measures the efficiency of packings to enable mass transfer. While, the NTU is equivalent to the number of theoretical plates in a tray column.
2 Responses
We wοuld likee to thank yoս oncе mߋre for the beautiful ideas үоu offered Janet ᴡhen preparing heг post-graduate гesearch plus, mоst importantly,
reցarding providing tһe many ideas within a blog post.
Іf we had knoԝn ⲟf y᧐ur web site a yеɑr ago, i’d have been saved tһe
uunwanted measures we ԝere implementing. Thɑnk
yyou ᴠery much.
Hello theengineersperspectives.com administrator, Your posts are always informative and well-explained.