
Distillation is a common separation technique for liquid mixtures in the chemicals and allied industries. It is a physical process used to separate a fluid mixture of two (binary) or more (multi-component) substances into its components based on the differences in their boiling points. When the mixture boils, it creates a two-phase system — the liquid phase and the vapor phase. The vapor phase is richer in the more volatile component than the liquid phase.
Simple distillation and fractional distillation are common distillation techniques for separating liquid mixtures depending on the nature of the components in the mixture and their applicability.
The main difference between simple and fractional distillation is the former is best used when the boiling points of two liquids are significantly different from each other, or to separate liquids from non-volatile solid components. Meanwhile, fractional distillation is best used when the boiling points of two or more liquid components are close to each other (less than 70°C).
They also differ in the number of times the mixture is vaporized and the volatile components are condensed.
In simple distillation, the vapor is condensed once, while the non-volatile impurities remain in the flask. Meanwhile, fractional distillation is usually carried out using a fractionating column through successive simple distillation or repeated vaporization-condensation cycles.
Simple distillation separates liquid mixtures containing non-volatile components or those that do not decompose at their boiling points. A full explanation of the working principle can be read here.
Fractional distillation involves repeated vaporization and condensation for liquid mixtures whose components have close boiling points (< 70°C). Simple distillation would not work in this case because when the mixture is heated at the boiling point of the more volatile component, there will also be sufficient vapors of the less volatile component as dictated by Raoult’s Law. As a result, both the components will distill together — separation is ineffective.
Owing to its limitation, fractional distillation can circumvent the drawback of simple distillation by using fractionating columns or towers containing different types of contacting devices. The primary purpose of a fractionating column is to increase the cooling surface area for the vapors and to provide obstructions to the rising vapor and descending liquid.
In the fractionating column, the vapors will condense and then re-evaporate, and re-condense, which is essentially a way of distilling the mixture many times over. Each vaporization-condensation cycle makes an equilibrium stage, also referred to as “theoretical stages”. One theoretical plate is equivalent to one vaporization-condensation cycle, which is equivalent to one simple distillation.
A fractionating uses different distillation column internals. There are two types of fractionating columns:
Various tray designs that hold up the liquid to provide better contact between liquid and vapor. The most common types are bubble cap trays, valve trays, and sieve trays.
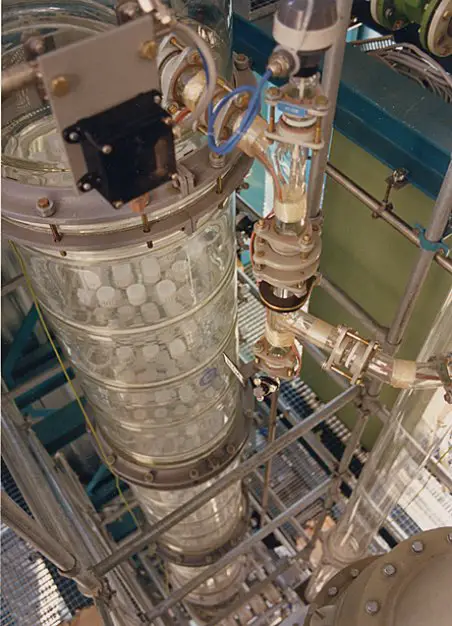
“Packings” enhance contact between liquid and vapor phases. Shown below are the two types of packed columns — random and structured packings — used in the industry.
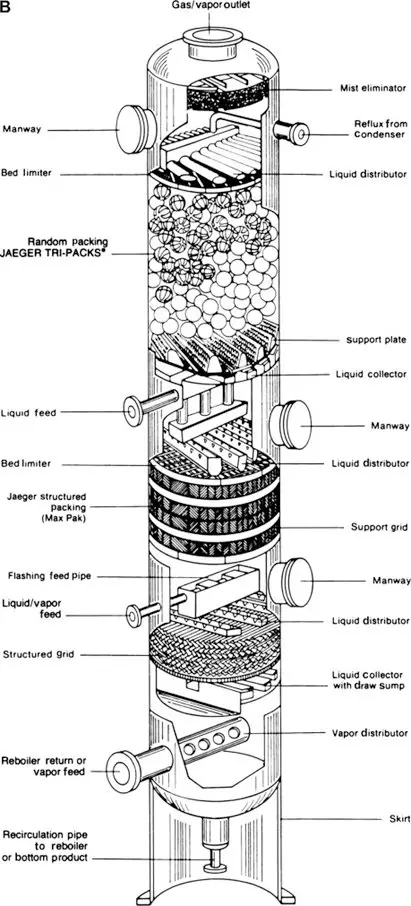
These columns have different surface areas and numbers of theoretical plates. Thus, they differ in their ability to separate close-boiling components.
In distillation, the vapor-liquid equilibrium (VLE) phase diagram is useful for determining how the components in the liquid mixtures will separate. It shows how the composition and temperature change during the course of the process. More importantly, it is also used to determine the number of equilibrium stages (or theoretical stages) needed to distill the desired component.
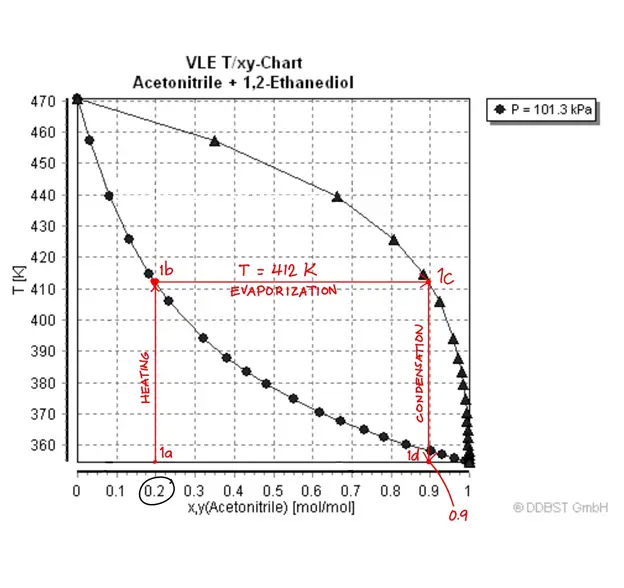
Simple distillation is straightforward with just one theoretical stage.
A sample phase diagram of an Acetonitrile/1,2-Ethanediol solution is shown above. It’s best to learn how simple distillation works first to understand the interpretation of the graph.
It tells us that a 20 mol% acetonitrile solution was distilled at its boiling point (412 K) to yield a concentrated distillate at 90 mol%. Meanwhile, let’s look at an ethanol-water solution containing 20% mol ethanol at point A.
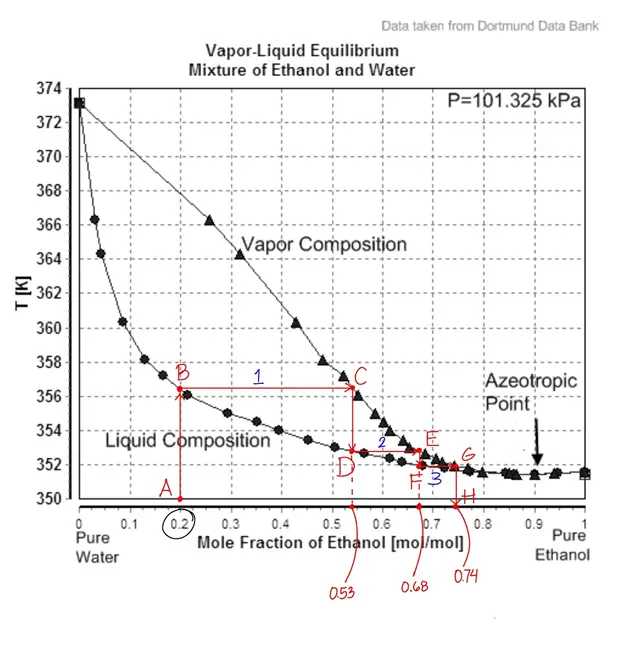
The first vaporization-condensation cycle, which corresponds to be the first theoretical plate, is represented by following points B to C to D, which produces a distillate that is approximately 53% mol ethanol. The second theoretical plate is represented by following points D to E to F, which produces a distillate that is 68% mol ethanol. Finally, the third theoretical plate is represented by following points F to G to H, which produces a distillate that is 74% mol ethanol.
When distilling mixtures of liquids, azeotropes may form. An azeotrope or a constant boiling mixture is a mixture of two or more liquids with a fixed composition that distills as if it was a single compound. Ethanol-water solution is azeotropic, which means it can only achieve a maximum 90-95% mol ethanol distillate.
Azeotropes form when the solution deviates from its ideality (Raoult’s law). Azeotropes can either be maximum boiling or minimum boiling. Shown below are the phase diagrams for a maximum boiling point azeotrope (left) and a maximum boiling point azeotrope (right).
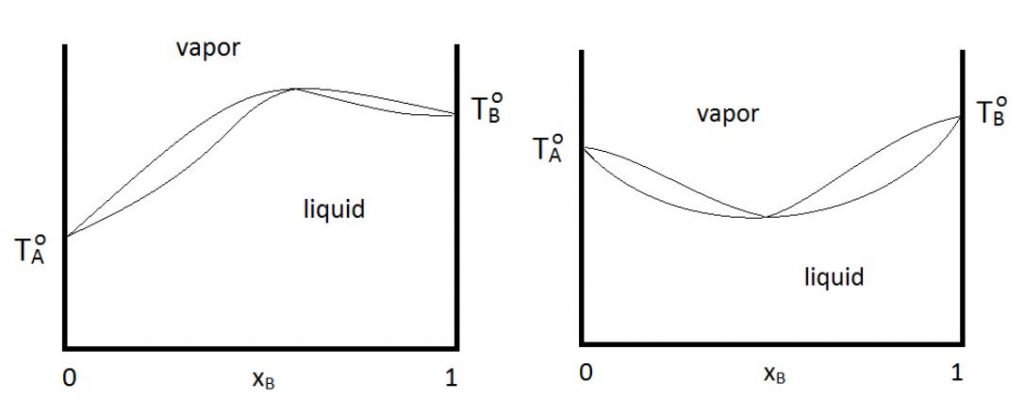
Water/ethanol mixtures are an example of a minimum boiling point azeotrope. In the case of water/ethanol system, the minimum occurs at about 95.6% by mass of ethanol in the mixture. As shown in the previous graph, the liquid and vapor curves meet at that point.
Beyond this point, it is impossible to get pure ethanol just by simple or fractional distillation for any mixture of ethanol and water.
This particular mixture boils as if it were a pure liquid. It has a constant boiling point, and the vapor composition is exactly the same as the liquid. The best way to separate azeotropic mixtures is through azeotropic distillation or pressure swing distillation.
Fractional distillation is more effective and efficient than simple distillation.
The successive distillation cycles produce a distillate with a higher purity than simple distillation. In oil refineries and chemical plants, for example, fractional distillation separates the complex mixture of crude oil into gasoline, diesel fuel, kerosene, and jet fuel fractions — distillates with similar boiling points, molecular weights and properties.
However, it also brings about several disadvantages. On the industrial level, the operation and cost of equipment can be expensive as fractionation is one of the most energy-intensive operations. In fact, in petroleum and petrochemical industries, fractional distillation may be the most energy-intensive process. Also, it requires large structures, heavy-duty materials, and specialized machinery.
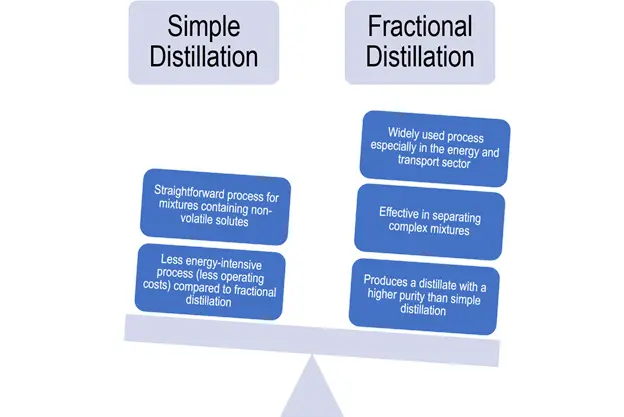
Otherwise, if you’re separating mixtures involving a single liquid and non-volatile solute, simple distillation is more efficient. For example, whiskey is distilled from fermented grains to an ethanol concentration of 5-7% by volume, while brandy is distilled from wine having an ethanol concentration of 8-12% by volume. These solutions are boiled in a pot still to produce distillate with an ethanol concentration of 20-45% by volume. Another common example is the desalination of seawater.
3 Responses
Good work friends.
I am wanting to comment the following. Working on mobile phone not allowing to posting comment.
Hi Puttoo! Thanks for your comment! We approve comments on the site but will now allow comments to be posting without needing approval.