There are different types of distillation depending on the nature of the components in the mixture to be separated and the operational conditions required to separate them. For instance, distillation of crude oil operates below atmospheric pressure to separate the heavier oils.
This type is called Vacuum Distillation, which this article intends to discuss in detail.
Vacuum distillation is the ideal separation method for liquid mixtures with very high boiling points (> 150 °C) and for components in the mixture that decompose when heated under atmospheric pressure. This unit operation is performed at a pressure below atmospheric level which is done by lowering the pressures in the column through a steam ejector.
To recall, the distillation process involves heating a multi-component solution above the boiling point of the most volatile component(s) to convert it from liquid to vapor phase, after which it is condensed and collected.
That being said, there are instances where the boiling points of the most volatile component is too high (> 150 °C) — like ethylene glycol (bp at 197 °C), for example. Higher boiling points require larger heating requirements which can be costly. The advantage of vacuum distillation is that at lower column pressures, the boiling point of the mixture also reduces. Take water in the diagram below, for example:
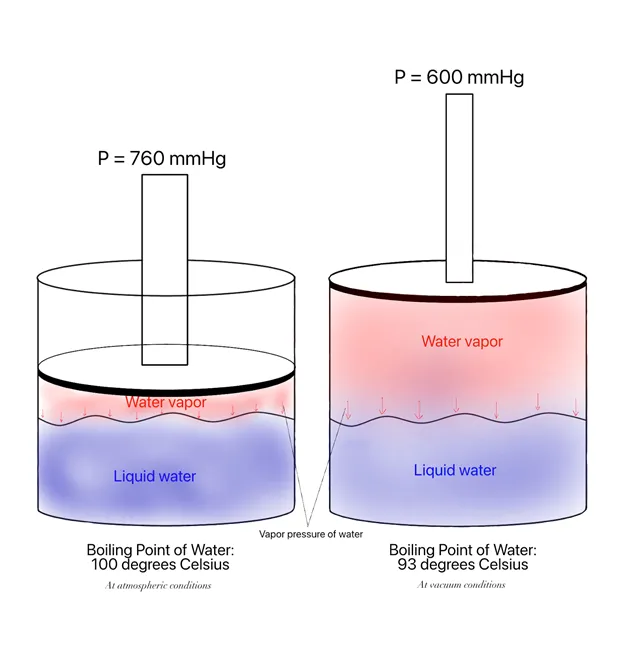
As soon as the vapor pressure of the substance in the system equals the vessel pressure, the mixture begins to boil. In atmospheric conditions, the boiling point of pure water is 100°C. When vessel pressure is reduced to 600 mmHg (vacuum), the boiling point of pure water is much lower at 93°C. This goes to show that it’s much faster to bring the mixture to its boiling point at vacuum conditions.
Essentially, a vacuum distillation system uses the same common equipment as that of fractional distillation, but under vacuum conditions. Thus, in addition to the column, distillation column internals, reboiler, and condenser, it must also be connected through-line with the steam ejector that creates the vacuum pressure.
A typical vacuum distillation process flow diagram is shown below.
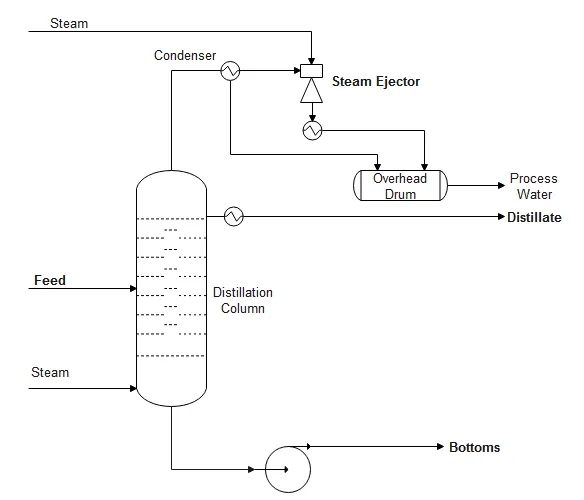
Vacuum distillation columns typically used in oil refineries have diameters ranging up to about 14 meters (46 feet), heights ranging up to about 50 meters (164 feet), and feed rates ranging up to about 25,400 cubic meters per day (160,000 barrels per day).
The steam ejector creates the vacuum by compressing the gases through a nozzle where vapors from the column are suctioned by a stream of medium or low-pressure steam.
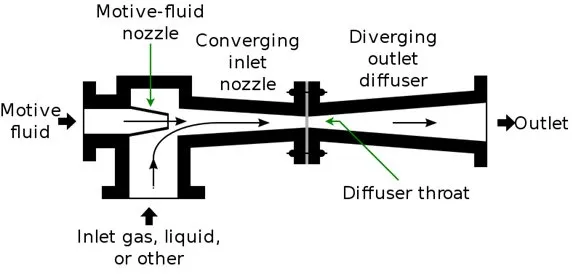
Following Bernoulli’s principle, the increase in gas velocity through the constricted opening of the nozzle (or any flow meter) results in a decrease in static pressure — allowing the column pressure to reach vacuum conditions. At the ejector outlet, gas partially condenses and the resulting liquid proceeds to the overhead drum.
Vacuum distillation has great industrial application value in various chemical and pharmaceutical industries — such as in alcohol vacuum distillation to preserve the flavor of spirits or in oil refineries to separate long-chain hydrocarbons
In oil refineries, fractional distillation of crude oil and petroleum products occurs at atmospheric pressure. The heavier oils at the bottom of the fractional distillation columns are fed through vacuum distillation units to increase the production of high-value petroleum products by further refining them .
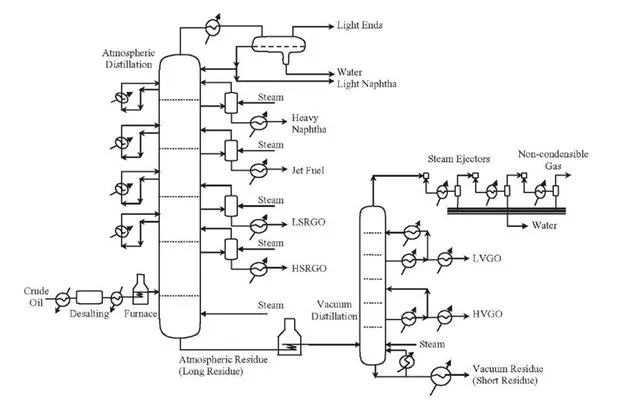
The schematic diagram of crude oil distillation shown above is a combination of fractional and vacuum distillation. The bottom residues of crude oil are further refined to light vacuum gas oil (LVGO), heavy vacuum gas oil (HVGO), and vacuum residues (e.g. paraffin wax and bitumen).
Meanwhile, alcohol vacuum distillation is best for distilling alcohol-water mixtures that might otherwise degrade through thermal damage. Distillation done at lower temperatures doesn’t “cook” the fermented grains, thus preserving its flavors. In the production of alcoholic spirits, vacuum distillation of gin under high vacuum, low temperature conditions (0.1 mmHg, -15°C) produces more distinct flavors and aromas.
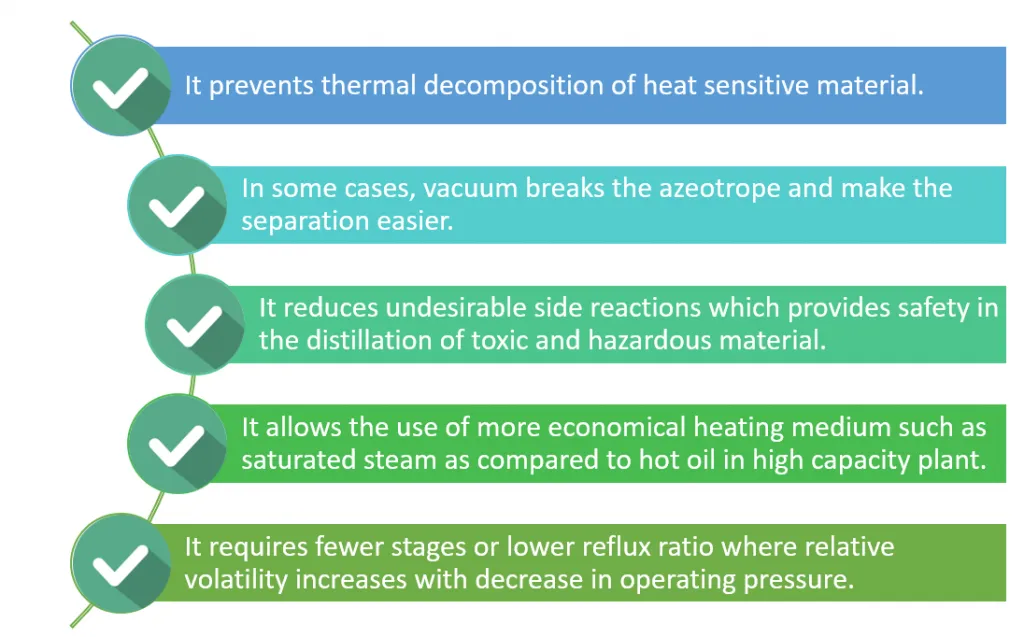
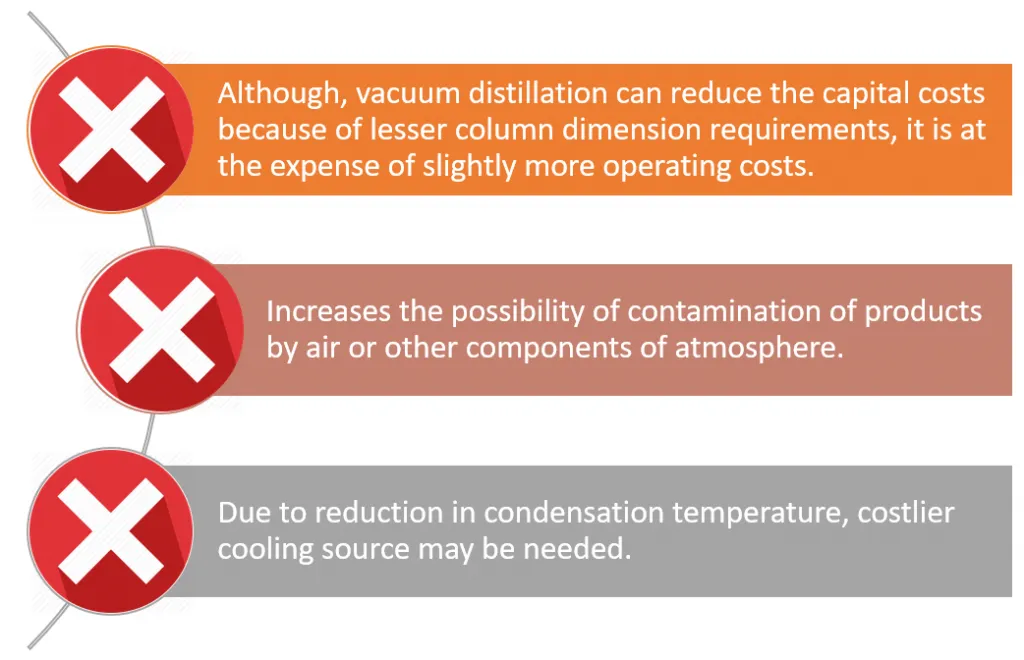
In summary, these are the main advantages and disadvantages associated with vacuum distillation.
1. Why is vacuum distillation better than simple distillation?
Vacuum distillation is better than simple distillation because it distills heavier materials at lower temperatures than those that would be needed at atmospheric pressure. Thus, avoiding thermal breakdown of the components.
2. What are some challenges with vacuum distillation?
Frequent occurrence of temperature fluctuations can affect the rate of evaporation. This is a major concern, especially in processes that require precision (e.g. when dealing with temperature-sensitive organic compounds or with reactive mixtures).