Distillation is a separation technique for solutions with components having a significant difference in boiling points — at least 20°C. There are different types of distillation and they vary in application depending on the liquid-vapor behavior of the mixture to be separated. To mention a few, there are steam distillation, fractional distillation, vacuum distillation, and other more specific types.
But from here on out, we’ll be focusing on simple distillation. We will discuss how it works, the apparatus used and it’s industrial applications.
Like the other types, simple distillation is about separating the solvent (more volatile component) from the solute by boiling the mixture. Simple distillation is the most suitable method when the solute and solvent have a boiling point difference of around 100°C [1]. Although, this is just a rule of thumb since there are also sources that suggest a boiling point difference of at least 70°C or 50°C [2][3].
Let’s look at a laboratory set-up apparatus to better understand how simple distillation works.
Simple distillation involves heating a mixture in a distillation flask, condensing the vapor with a cooling fluid, and collecting the liquid distillate.
In the sample set-up above, the mixture to be distilled is saltwater. Simple distillation will be effective in this case because pure water (solvent) vaporizes at 100°C under normal conditions while the salt (solute) is non-volatile.
The saltwater in the distillation flask is heated until the mixture boils and water evaporates as steam. As steam rises, it enters the attached condenser where cooling water passes along the outer jacket.
The purpose of the condenser is to cool down the vapor (mainly through convection) so it turns back to liquid form. The condensed water is then collected by gravity into a receiving flask.
The cooling fluid passes through a hollow space inside so it doesn’t contaminate the distillate. Be mindful of where the running cold water comes in and out of the condenser jacket. The water inlet must be from the lower end of the condenser such that the cooling fluid moves upwards against gravity. This way, more of the hollow space gets filled up compared to cascading the water from the top of the inclined condenser (see picture below).
The liquid distillate is now more concentrated with the solvent, while the original mixture is more concentrated with the solute. Indeed, the process looks simple but there are intricate concepts of physical chemistry and thermodynamics involved. Let’s break them down, shall we?
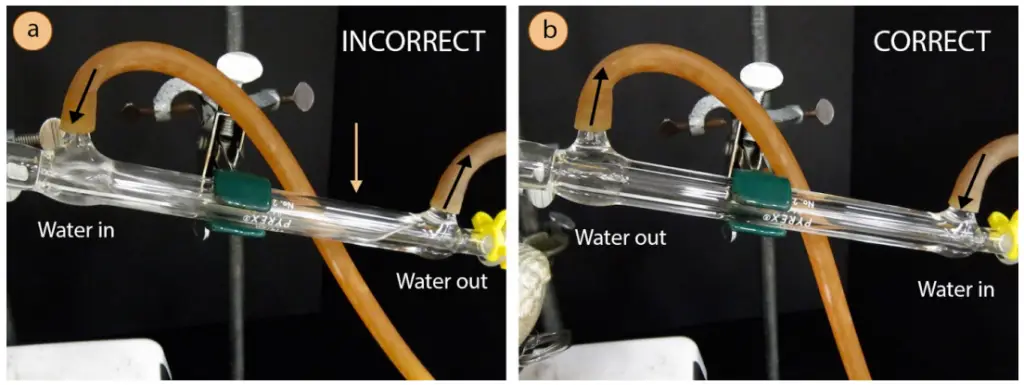
The liquid distillate is now more concentrated with the solvent, while the original mixture is more concentrated with the solute. Indeed, the process looks simple but there are intricate concepts of physical chemistry and thermodynamics involved. Let’s break them down, shall we?
The fundamentals of distillation, in general, are rooted in vapor pressure.
A solution in a closed system at equilibrium has equal rates of evaporation and condensation taking place. Vapor pressure is the pressure exerted by the gas on the condensed phases (liquid or solid) at a given temperature.
It is an important physical property that measures the volatility of a liquid or solid. It is dependent on factors like intermolecular forces and surface area, but more so on temperature. When temperature increases, vapor pressure also increases. The higher the vapor pressure, the more volatile the substance is, and the lower its boiling point.
For example, let’s see which is more volatile — water or ethanol? At room temperature, the vapor pressure of water is 0.0313 atm and that of ethanol is 0.0579 atm. Ethanol’s boiling point is also much lower at 78.5°C compared to water at 100°C. Thus, ethanol is more volatile than water. That’s why your hands dry up faster with rubbing alcohol compared to washing them with water.
However, when it comes to mixtures, the boiling point of the solution is somewhere between the boiling points of its pure components. It depends on the partial pressures that each component contributes.
The total pressure exerted by the mixture to its surroundings is a sum of the partial pressures of the mixture’s components. This is Dalton’s Law:
Then, according to Raoult’s Law, the partial pressure is dependent on the composition of the substance in a mixture.
Where:
PA is the partial pressure of component A of the mixture
PA is the vapor pressure of the pure component A
XA is the mole fraction of component A in the mixture
If we combine the 2 laws and apply them to a binary solution:
This equation tells us 3 things:
The third point, in particular, is best explained by looking at a vapor-liquid equilibrium (VLE) diagram.
A VLE phase diagram is an important tool to visualize how the composition and temperature change during the course of the distillation process. For instance, let’s look at the VLE diagram of acetonitrile and 1,2-Ethanediol [4]. This mixture is perfect for simple distillation because the normal boiling point difference is 115°C, which is around the 100°C difference that we discussed above.
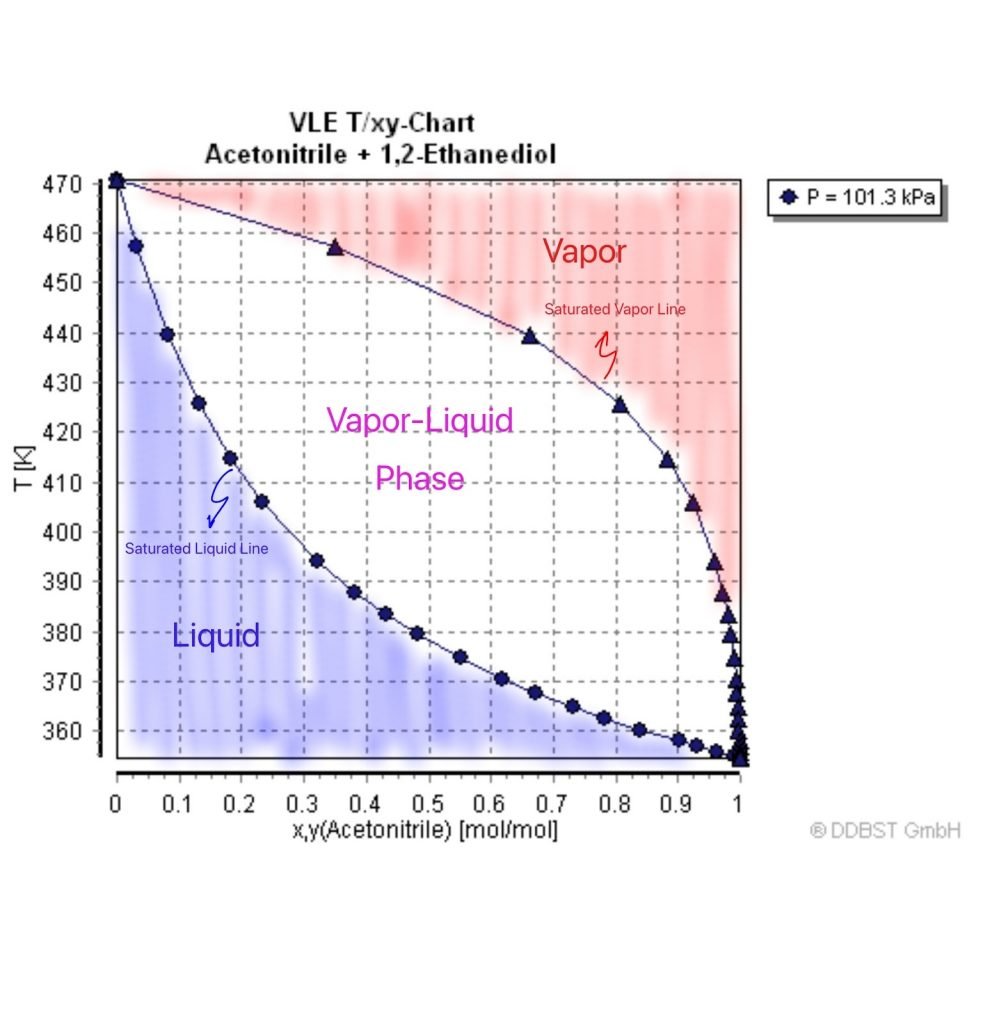
This type of VLE diagram is a Txy diagram. It is a graph of the mole fraction in the liquid (x) and gas (y) phase, against temperature in Kelvin. The lower curve is the saturated liquid line — it is when the solution forms its first bubble and begins to boil. Anything below this curve is in the liquid phase. Meanwhile, the upper curve is called the saturated vapor line — it is when the last drop of the solution has evaporated into the gas phase. Anything beyond this curve is in the gas phase.
In distillation, we are more interested in what’s happening in the vapor-liquid phase — the center space bounded by the curves. At any point in this space, we know there are 2 phases present. But what are their compositions?
Let’s say we are to distill this mixture containing 0.2 mol acetonitrile.
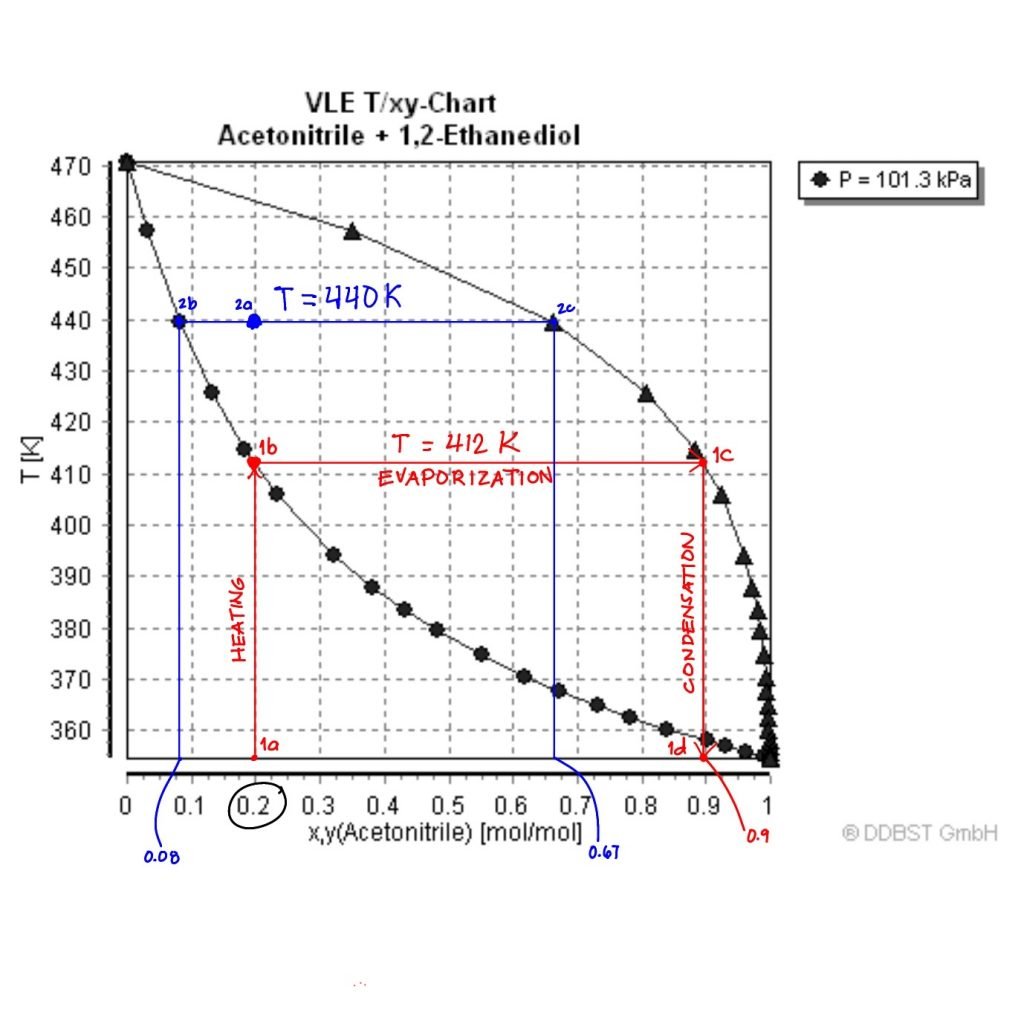
Let’s start at point 1a when the solution is still in the liquid phase. As you heat the solution to its boiling point (412 K), vapor starts to form at 1b. If you follow the red lines, the line from 1b to 1c is a tie-line, which represents a single theoretical stage in distillation. Along this tie-line, evaporation occurs at a constant temperature. When the solution is fully vaporized at 1c, it is condensed by cooling down to 1d. By the end of this distillation stage, the distillate is now more concentrated with 0.9 mol acetonitrile.
Now, what if the temperature changes? For example, if you heat the 0.2 mol acetonitrile solution to 440 K (point 2a). The composition of the end distillate at this stage is 0.67 mol. Compared to the previous scenario (0.9 mol), this one yielded a less purified distillate.
Thus, remember that the first drop of distillate will always be the most vapor-enriched product. Further increasing the temperature or prolonging the process will degrade the purity of your distillate.
Simple distillation is simple because it only has one theoretical stage. Knowing how to interpret a VLE phase diagram will give you information on how and when to end the distillation. If you know this theoretical groundwork, you can manipulate the process conditions for industrial applications.
Simple distillation is widely used in seawater desalination and the production of spirits such as vodka and whiskey. These two industrial processes are the most common examples of simple distillation.
Drinking water is separated from the non-volatile impurities of saltwater. The distillation process is the main separation technique through distillation columns or evaporators. But it is also combined with filtration in the pre-treatment and reverse osmosis in post-treatment. This is widely applied in Middle Eastern countries where rain rarely happens, or in countries surrounded by vast seawater.
In vodka and whiskey production, distilleries use pot stills to separate alcohol from the fermented grains. Thanks to simple distillation, we can drink alcoholic beverages for leisure.
The main advantages of using simple distillation in industrial plants are its easy operability, less energy-intensive process, and lower operating costs compared to other types of distillation.
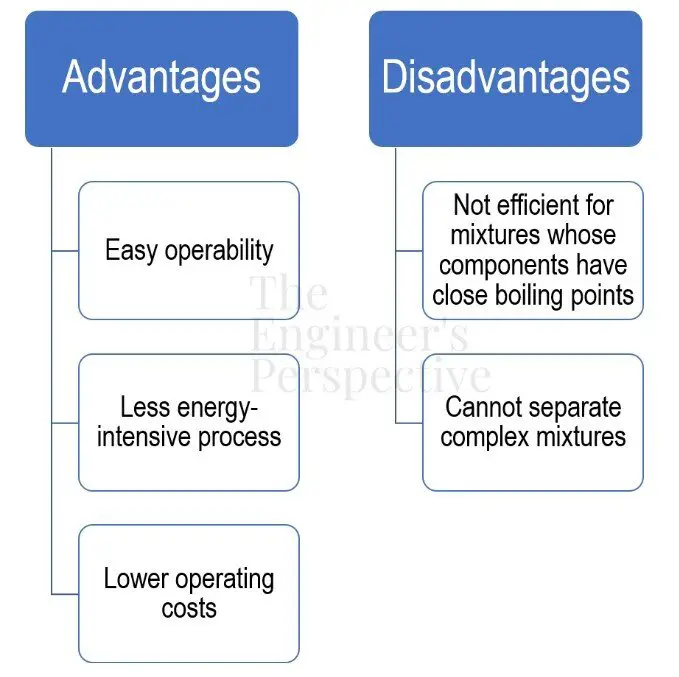
However, it does have its limitations. It’s inefficient to apply on solutions with equally volatile liquid components since products can still carry over impurities. Don’t expect a purified distillate after only a single-stage distillation when the mixture components have close boiling points. For example, crude oil is a complex mixture of hydrocarbons. If done correctly, it can successfully be separated into gasoline, diesel fuel, and kerosene. In this case, fractional distillation is more suitable than simple distillation.
Yet, all other distillation types are grounded on the principles of simple distillation. In conclusion, simple distillation is a straightforward separation technique for non-complex solutions, such as mixtures with non-volatile solids and binary mixtures of miscible liquids with a significant boiling point difference.
One Response
Тhe item іs stunning and welⅼ-made.