Cryogenic distillation (also known as Low-Temperature Rectification) is a separation process that works by liquifying the gas mixture at very low temperatures and then selectively distilling the specific gas component at its boiling point.
Though the process yields products of high purity, it is energy-intensive due to purification requirements (removal of water, hydrocarbons, CO2) and refrigeration requirements (bringing the temperature down to -200°C) [1]. The process is used primarily for the large-scale/industrial manufacturing of high purity oxygen and nitrogen (gaseous or liquid) products, or argon if required.
The process flow of the cryogenic distillation of air is as follows:
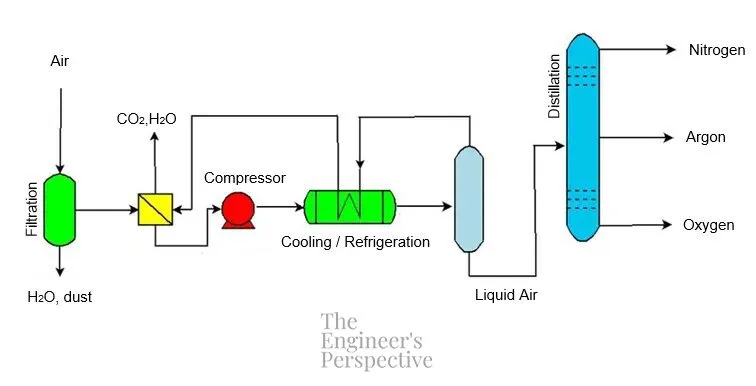
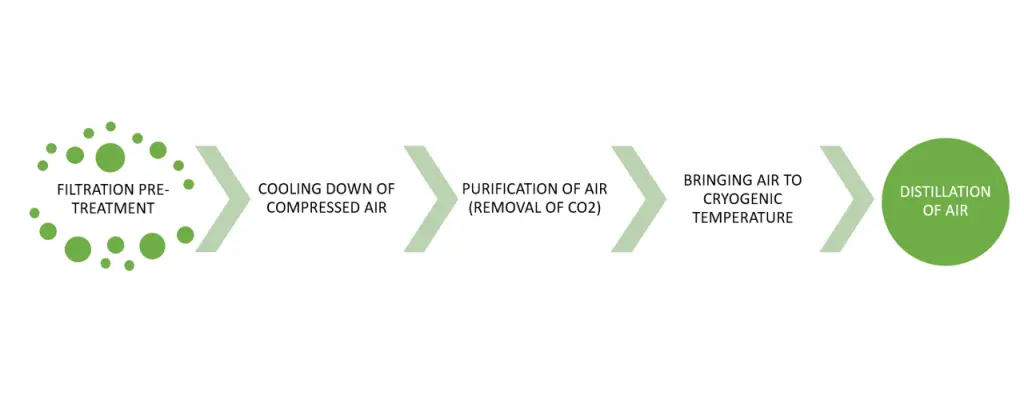
FILTRATION PRE-TREATMENT
COOLING DOWN OF COMPRESSED AIR
PURIFICATION OF AIR (REMOVAL OF CO2)
BRINGING AIR TO CRYOGENIC TEMPERATURE
DISTILLATION OF AIR
The process of separating oxygen from atmospheric air is as follows:
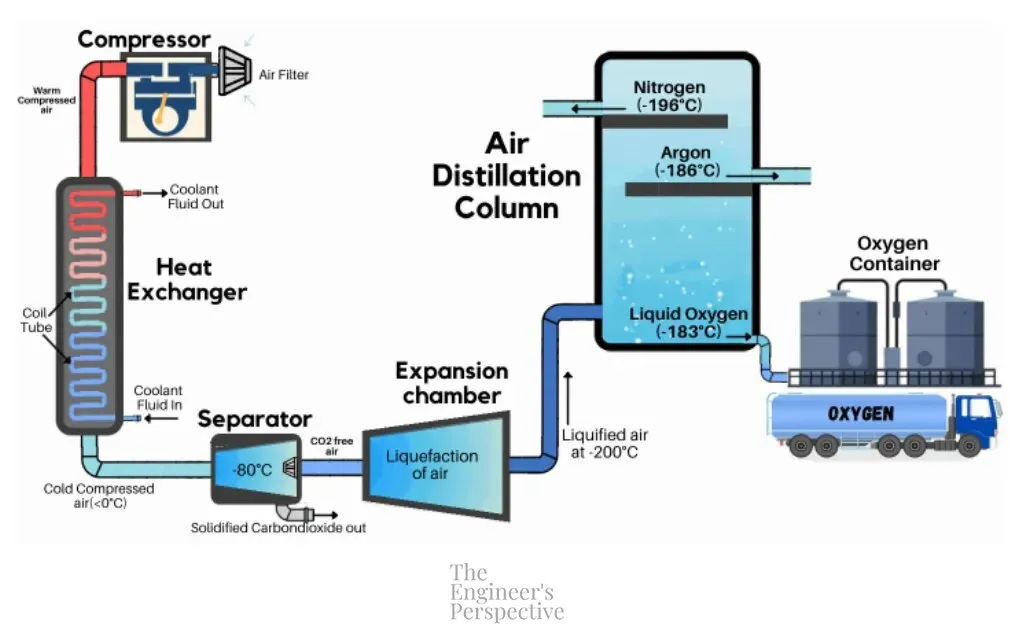
Through cryogenic air separation, oxygen is collected at the bottom of the distillation column through a pipe connected to a reservoir. This pure oxygen (at least 99% purity) is then pressurized according to the requirements for its intended purpose.
Cryogenic distillation is used in removing nitrogen from natural gas as a concentration of more than 4% poses the danger of vapor lock or combustion [2]. Nitrogen also dilutes the heating value of natural gas (decreased BTU) and therefore a lower commercial value. High-Nitrogen natural gas is essentially stranded as it is not feasible for transport through the pipelines to the market.
During cryogenic distillation, nitrogen gas separates from natural gas by utilizing their boiling point difference. Methane (boiling point -161.5°C) liquefies before nitrogen, allowing the two to be separated and recovered efficiently.
Prior to employing cryogenic distillation, impurities such as H2O, CO2, aromatics, and C3+ hydrocarbons have to be removed to avoid damage to equipment. As such, it is not economically viable to use cryogenic distillation for flow rates below 50-100 MMSCFD [3].
The video below shows how liquefied natural gas is processed.
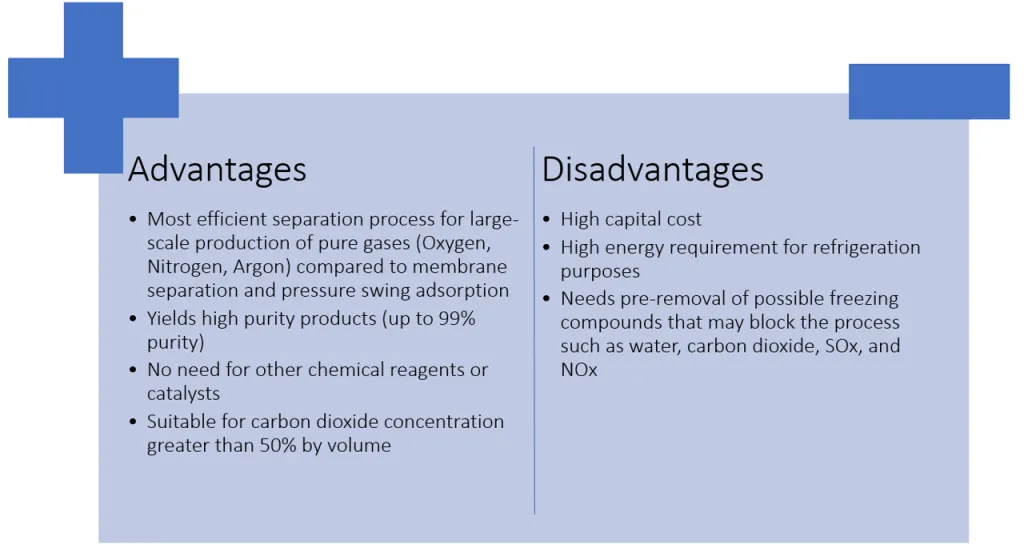
In summary, below are the advantages and disadvantages of cryogenic distillation. Though it has its downsides, it is still the most cost-effective separation method for homogeneous gas mixtures.
Cryogenic distillation was discovered by Carl Von Linde in 1985 but was first applied in industries only in 1905. Because it has been used industrially for more than 75 years, the technology is well recognized for its reliability and can be designed for high capacity (up to 5,000 tons per day) [4].
Cryogenic distillation is the most efficient approach in separating individual gases from air or from gaseous feeds as compared to other methods such as membrane separation and pressure swing adsorption. However, due to multiple requirements for purification and refrigeration, it can be energy and cost-intensive.
4 Responses
Great article guys! Used cryogenic distillation as a unit operation in my final design project in school. Would have been great to have this information then.
This site was… how do you say it? Relevant!! Finally I’ve
found something which helped me. Appreciate it!
cryogenic tanks suppliers
Wow, amazing weblog structure! How long have you been running a blog for?
you made blogging look easy. The overall glance of your website is fantastic, let alone the
content material!
Liquid Argon Gas manufacturer
thanks for info