
Simple Distillation vs. Fractional Distillation
Difference between simple vs fractional distillation is the number of times the mixture is vaporized and the volatile components are condensed.

Difference between simple vs fractional distillation is the number of times the mixture is vaporized and the volatile components are condensed.

Simple distillation is the most suitable method when the solute and solvent have a boiling point difference of around 100°C
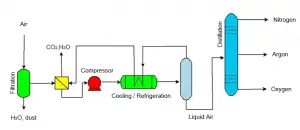
Cryogenic distillation is a process that works by liquifying the gas mixture at very low temperatures.
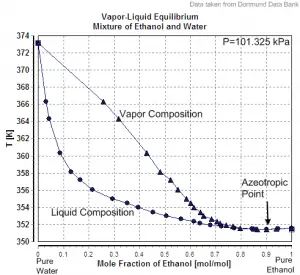
Azeotropic distillation separates azeotropic mixtures by adding an entrainer that changes the relative volatility of the substances.

Steam distillation, or steam stripping (depending on its application) is a distillation separation technique which utilizes steam as a carrier vapor to separate temperature-sensitive volatile components in a solution at a much lower temperature than its boiling point.

Solid-Liquid Extraction is a separation technique which separates a solute attached to an insoluble solid phase via an extraction solvent.