
Chemical Engineer
Liquid-Liquid Extraction (LLE) has common applications in the Chemicals, Wastewater and Petrochemical industries for extracting valuable substances or removing contaminants from a feed stream.
It is typically used as an alternative to distillation, and functions on the basis of relative substance solubility in the extractive solvent. It is also seen as a suitable replacement for distillation as an extraction process in the case of high energy requirements and process design economics (column sizing) [1].
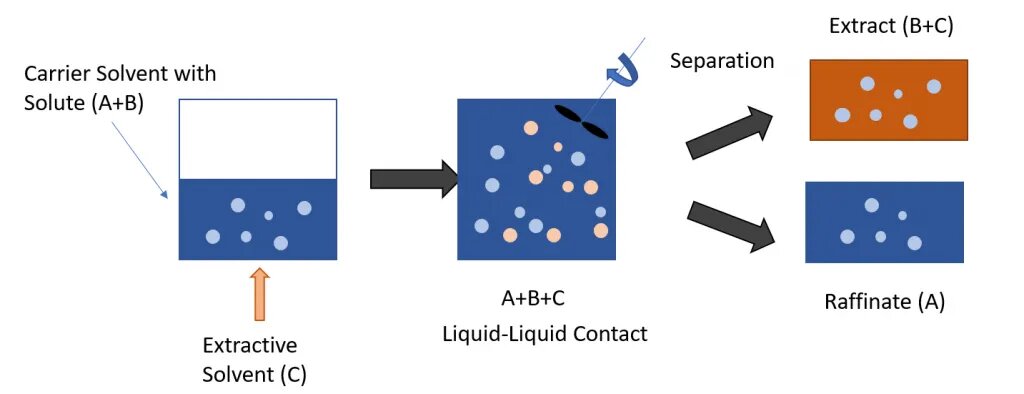
Liquid-Liquid Extraction is a physical separation process involving the mass transfer of the desired solute typically from an aqueous carrier solvent phase to the organic extractant solvent phase. It is also sometimes a chemical process depending on the interactions between the solvent and feed stream (ion exchange between phases or formation of chemical intermediates).
Other examples of solvent extraction include Solid-Liquid Extraction, otherwise known as leaching.
There are typically three stages involved in a liquid-liquid extraction mechanism:
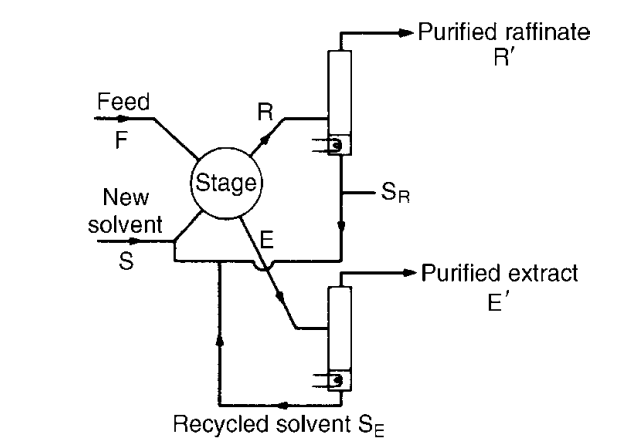
In industrial applications, the two resulting streams following the contact stage of the process are the extract (E) and the raffinate (R) streams. The extract stream, as indicated by its name, is rich in the extraction solvent and the desired components/solute. The raffinate stream is the residual liquid following the separation of phases. It is composed of a weaker concentration of the desired solute and the extraction solvent.
Ultimately, LLE is driven by these three phenomena [2]:
The equilibrium phase is dictated by the chemical properties of the feed mixture although separation between phases is dictated by the relative affinity/degree of miscibility of the desired product to either solvent.
The extraction solvent is typically non-polar(organic) whilst the extraction solvent is polar (aqueous). The concentration difference across both phases also determines the solute’s rate of mass transfer to the extracting solvent.
As a system approaches a state of thermodynamic equilibrium, it seeks to minimize its chemical potential or Gibbs Free Energy, becoming more stable.
The two categories of miscibility (using the naming conventions outlined above) which dictate the equilibrium state of the liquid-liquid extraction system are:
The phase equilibrium of the components in liquid-liquid extraction is graphically represented by a ternary diagram.
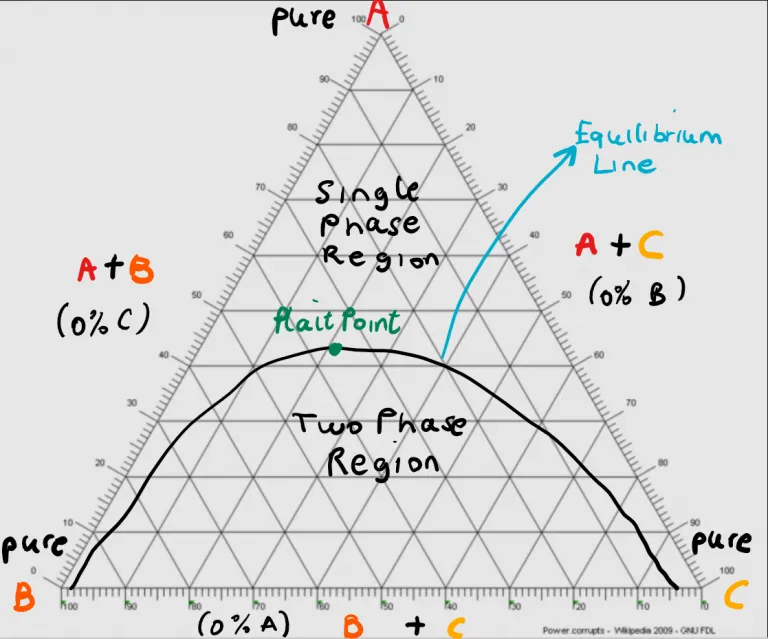
Here are a few important things to note about reading ternary diagrams:
These diagrams are also used to complete mass balances to determine the flow rates and compositions of the extract and raffinate streams as well as the number of theoretical stages required for extraction. Explanation of this methodology is out of the scope of this article.
The degree of separation in a LLE process is typically quantified by what is called the partition coefficient or distribution constant which is defined as a ratio of the solute (desired product) in the two phases (extract and raffinate).
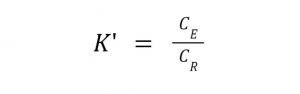
where:
K’ = Distribution/Partition Coefficient
CE= Concentration of Solute in Extract/Organic phase
CR= Concentration of Solute in Raffinate/Aqueous phase
The ratio is only correctly applied if both the carrier and extraction solvent are immiscible at equilibrium and typically holds if the concentrations are small and no chemical reaction takes place.
The three main types of vessels used in the continuous liquid-liquid extraction process include:
Industrial applications of liquid-liquid extraction are either batch or continuous processes. In In batch processes, the first contact and separation phases of the process occur within the same column.

In a continuous process, contact between a feed stream containing the carrier and extraction solvent happens in multiple stages whereas the separation and recovery phases in another processing unit via gravitational settling and another extraction process such as distillation respectively.
In hydrometallurgy, liquid-liquid extraction is used to extract pure elements such as copper from their aqueous ores using organic solvents. In its petrochemical application, acidic gasses such as CO2 and H2S generated in fluid catalytic crackers are extracted from liquefied petroleum gas (LPG) using amine solvents [1].
Another common application of liquid-liquid extraction is in the recovery of antibiotics such as penicillin from its aqueous fermentation broth using oxygenated organic solvents [1].