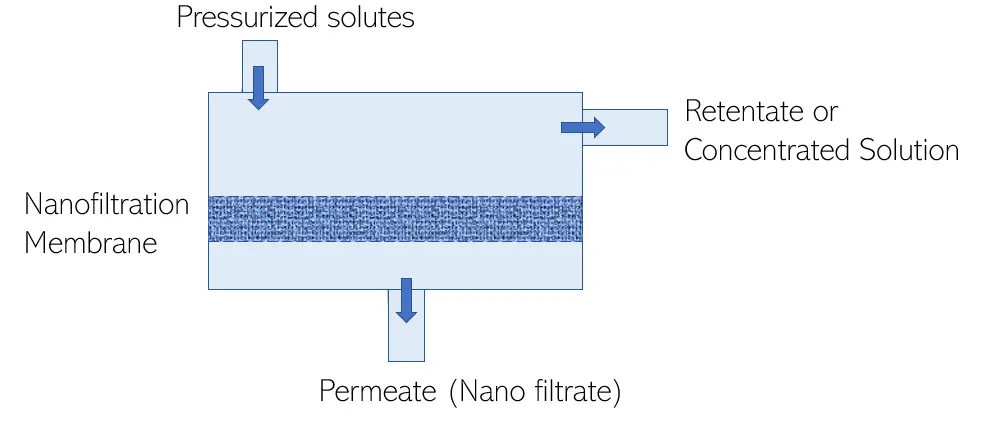
Nanofiltration is a specialty pressure driven membrane process where in addition to size exclusion as a removal mechanism, the surface charge of the membrane impacts the permeability of charged components.
Nanofiltration membranes will reject dissolved solutes with sizes around 1 nm or 10 angstroms, which is why it is called ‘nano’ filtration’. Furthermore, the molecular weight cut-off is generally around 400 Da plus or minus 100 Da. Nanofiltration membranes can exhibit a slight positive or negative charge, and that charge can either repel or attract charged ions in the feed stream which directly affects their permeability.
Relative to the other membrane processes, nanofiltration operates between ultrafiltration and reverse osmosis.
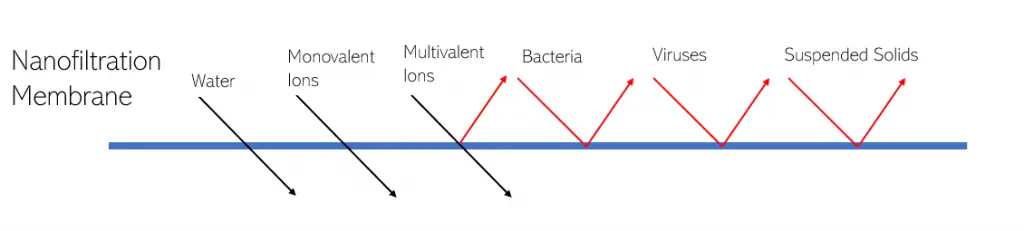
The nanofiltration membranes will allow water, and the majority of monovalent ions to pass through the membrane, while retaining some monovalent ions, bacteria, viruses and suspended solids. From the multivalent ion category, the nanofiltration membrane can effectively remove divalent ions such as calcium ions or magnesium ions.
Additionally, they can also effectively reject herbicides, antibiotics, suguars, dissolved organics, insecticides/pesticides, latex emulsions, and nitrates.
Transport mechanisms refer to the mechanism by which the feed stream is able to traverse through the membrane. In the nanofiltration membrane, there are 2 main theories that are adopted.
Theory 1 describes the transport mechanism through which the nanofiltration membrane uses the principles of sorption and desorption. It describes the preferential sorption of water through the membrane, and the desorption of multivalent ions which cause the exclusion of charged solutes which may even be smaller than the membrane pores themselves.
Theory 2 describes the transport mechanism by defining the nanofiltration membrane as a porous film where water and solute (ion) dissolve. The solute then moves based on the concentration gradient, and the transport of the solute depends on the convection and hindered diffusion.
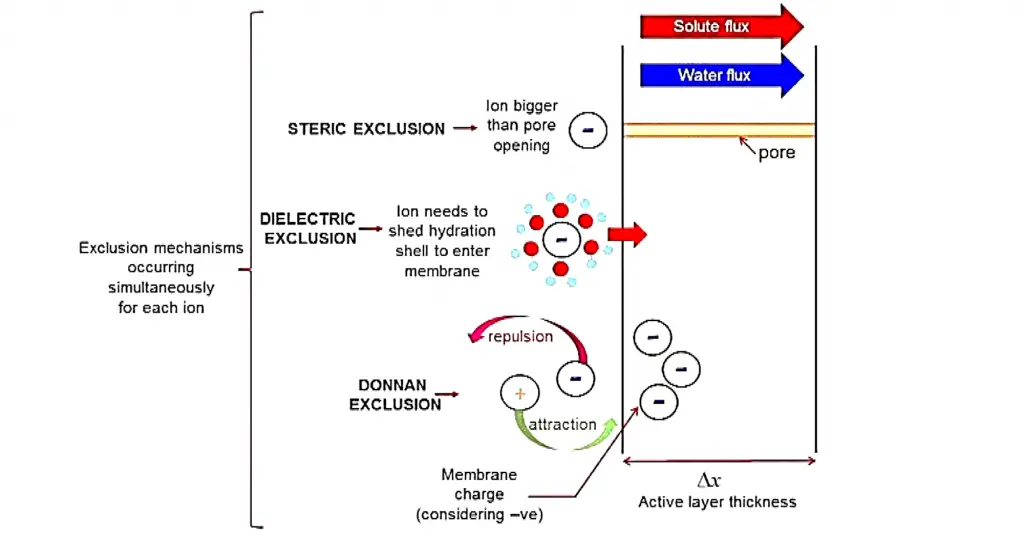
Just like the other pressure driven filtration methods, nanofiltration utilizes size exclusion to separate particles and other components based on their size. However with regards to ion removal, there are 3 exclusion methods that come into play namely:

The donnan exclusion phenomenon has a pronounced effect with nanofiltration membranes when compared to other pressure driven membrane processes such as microfiltration or ultrafiltration since we are mostly dealing with ions.
Donnan exclusion describes the charged ion distribution near the nanofiltration membrane. Due to the slightly charged nature of the nanofiltration membrane, solute ions with an opposite charge relative to the membrane will feel a slight attraction, and solute ions with the same charge will repel.
Furthermore, at the surface of the membrane, there exists a distribution of the positive and negative charges which causes additional separation.
Due to the dipole moment of water and the charge on the membrane, the water molecules become polarized. This polarization leads to a decrease in the dielectric constant inside the membrane pore which makes it less favorable for a charged solute to enter, which results in exclusion.
Simply put, steric exclusion is a size exclusion method where the ion is bigger than the pore opening which does not allow the ion to pass through the nanofiltration membrane.
Below is a schematic that shows the primary rejection mechanisms that prevent solutes from entering nanofiltration membrane pores.
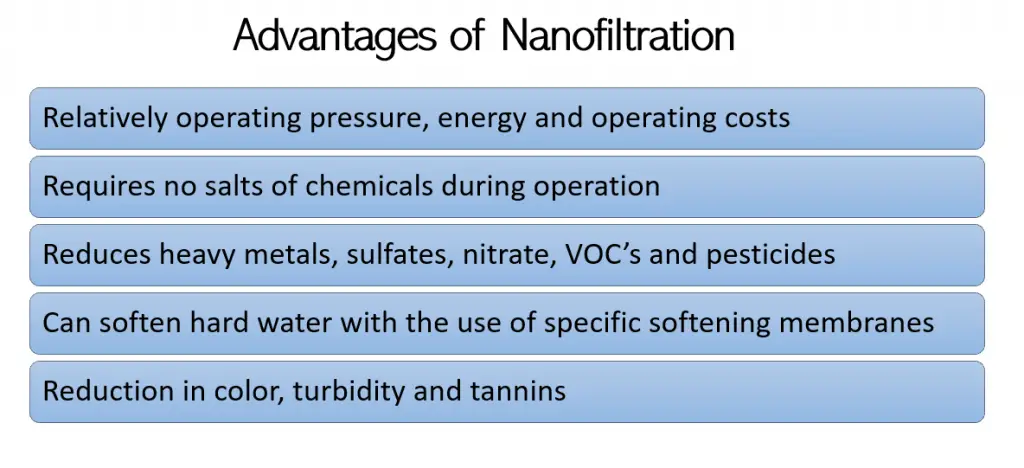
The following are advantages of nanofiltration:
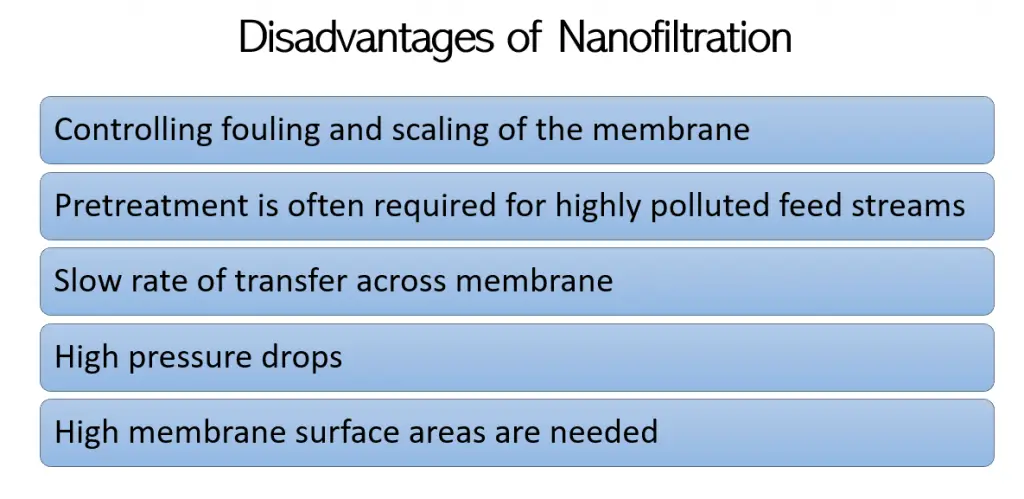
The following are disadvantages of nanofiltration:
The 4 main parameters that affect the performance of the nanofiltration membrane are:
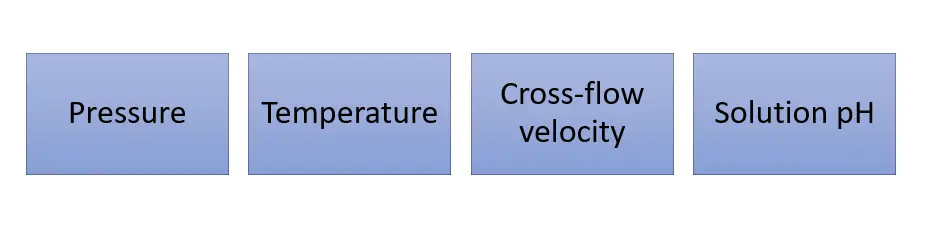
The pressure is the driving force responsible for a nanofiltration membrane process. An effective driving pressure is the applied hydraulic pressure minus the osmotic pressure applied on the membrane by the solutes.
The nanofiltration flux increases with an increase in temperature. This is because an increase in temperature decreases the viscosity of the fluid.
The nanofiltration flux increases with an increase in cross-flow velocity due to the more efficient removal of the fouling layer on the membrane surface.
However, there exists a maximum cross flow velocity where if this is exceeded, there may be premature failure of the membrane and module.
Charged sites on the nanofiltration membrane surface vary with pH. At a neutral pH, the sites are negatively charged, and at an acidic pH, they lose their charge.
One of the main purposes of nanofiltration is to separate monovalent ions from multivalent ions. This allows applicability across a wide range of industries, the main ones being wastewater, food and beverage, textiles and dyes and pharmaceuticals.
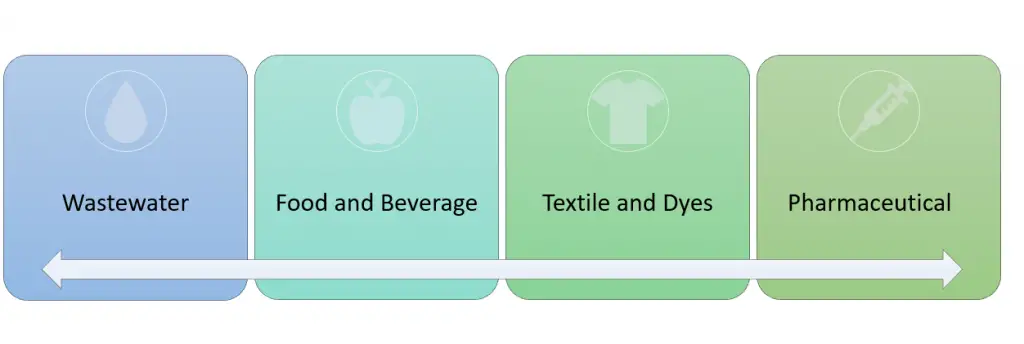
There are many applications for nanofiltration membranes in the wastewater industry – both domestic and industrially.
Within the wastewater industry, brine recovery and leachate treatment are two important examples. The process of brine recovery involves filtering out high concentrations of sulfates from the water stream while allowing salt to pass through. Leachate is essentially a highly contaminated liquid which is also treated by nanofiltration to remove pollutants and decrease organics, chemical oxygen demand, and turbidity.
In the food and beverage industry, there are a whole plethora of applications within different food groups such as sugars, fruits and vegetables, dairy and grain products. For example, within the dairy industry, demineralized whey is highly sought after due to its high nutritional value and better taste. Nanofiltration is used as one option to accomplish a partial demineralization of whey and retain the required mineral level from the pasteurized whey.
Textiles and dyes is another major industry that utilizes the nanofiltration membranes in order to help with dye penetration removal, and concentration of dyes and agents. For example, optical brightening agents are chemicals added to clothing to give the appearance of a lighter and brighter finish. Nanofiltration membranes can be used as a high efficiency alternative to increase the concentration of these synthetic agents in order to meet the specifications.
Within the pharmaceutical industry, production of antibiotics and concentration are important applications. Fibryga is a concentrate of fibrinogen, and is used to treat acute bleeding. Now in order to get the highly purified fibryga, nanofiltration is used with 20nm openings to ensure proper treatment for patients with congenital fibrinogen deficiency.
When using nanofiltration in industry, there are a couple key challenges that arise and must be addressed. Scaling and fouling of the membranes are the main issues.
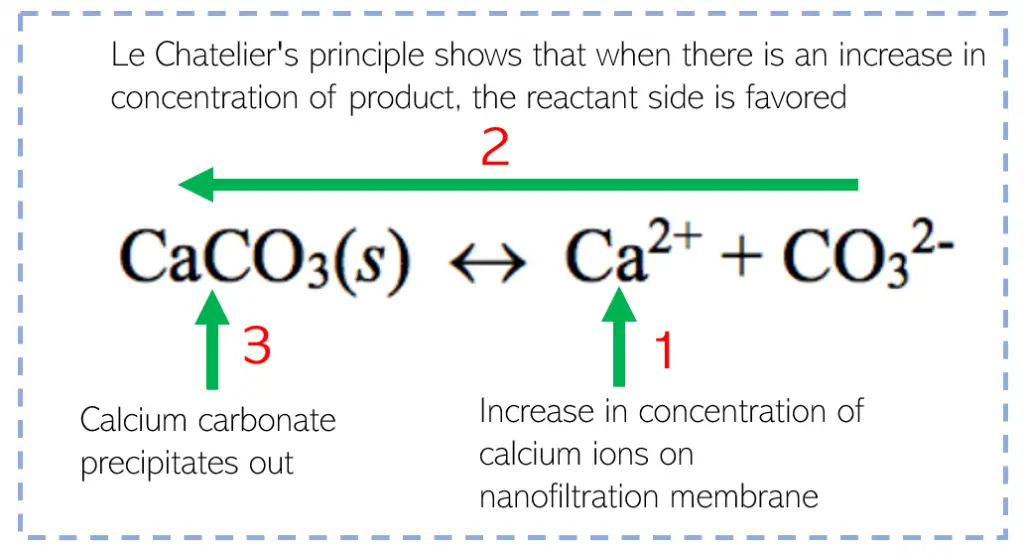
Calcium, in particular, has the ability to foul nanofiltration membranes. Due to this, the reverse dissolution reaction will be favored which causes the calcium carbonate to be precipitated out onto the membrane. This leads to scaling of the membrane which may potentially plug up the membrane.
Fouling of the membrane can come from organic matter which can be adsorbed onto the membrane surface which can cause rejection, and greater pressures for water flux.
Biofouling is another issue which involves the undesired development of microbial layers on the surface of the nanofiltration membrane. Once conditions are met to allow the microbes to thrive, microbial colonization and growth can lead to solute rejection and high pressures.
One of the most effective ways to prevent scaling is to pass the feed stream through a pre-treatment process which can remove the ion that causes the scaling before it gets to the nanofiltration membrane. Filtration of particulates such as organic foulants is also a good idea to prevent a deposition on the membrane.
The addition of chemicals is also a common operational solution where antiscalants can be added to prevent precipitation. If we look at our calcium example above, adding a ligand which makes the calcium more soluble will decrease the free calcium in solution even though the total calcium in solution is the same. This will cause the reaction to not be forced to the left to produce the calcium carbonate precipitate.
Another way to prevent scaling is to inhibit crystal growth. In the precipitation process there are multiple stages such as nucleation, crystal growth and agglomeration, and finding ways to inhibit steps of this process help with the scaling that occurs from fully precipitated crystals.
The math behind the nanofiltration membrane gets more complex since we are introducing the electrical interaction between the charged membrane surface and the ions in solution, which will be determined by the Donnan exclusion.
Understanding the detailed derivations are beyond the scope of this article, however we will provide a high level overview of the equation for determining the individual flux of components passing through the nanofiltration membrane.
In our derivation for ultrafiltration volumetric flux, we were able to assume that the osmotic pressure was negligible on the solvent flux. However, when looking at nanofiltration, we cannot do this because this term will have an effect on the solvent flux.
Furthemore, when we consider donnan exclusion, we have to determine the flux of electrical charges through the surface area of the membrane. This total electrical charge flux is a sum of the charge number, Faraday constant, and charges of the individual ions in solution.
If we assume that the feed solutions are relatively dilute, we end up with the following equation for calculating the flux of individual components passing through a nanofiltration membrane which contain either positive or negative charges:
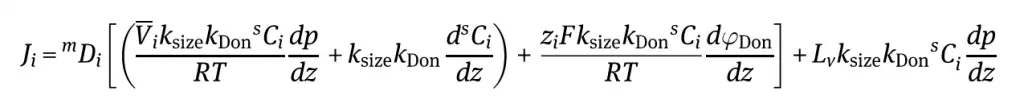
Nanofiltration is primarily used for getting rid of ions in solutions, is generally used with a pretreatment method.
Nanofiltration membranes do not remove salt effectively, however reverse osmosis membranes can be used to separate more than 99% of salt from water.
As the name suggested, the “nano” filtration membrane has a pore size of about 1 nanometer which is equal to 1 billionth of a meter.
To give you some perspective on how small this is, a typical sheet of copier paper is just over 100,000 nm thick. Another example is a DNA molecule which is about 2.5 nm wide.