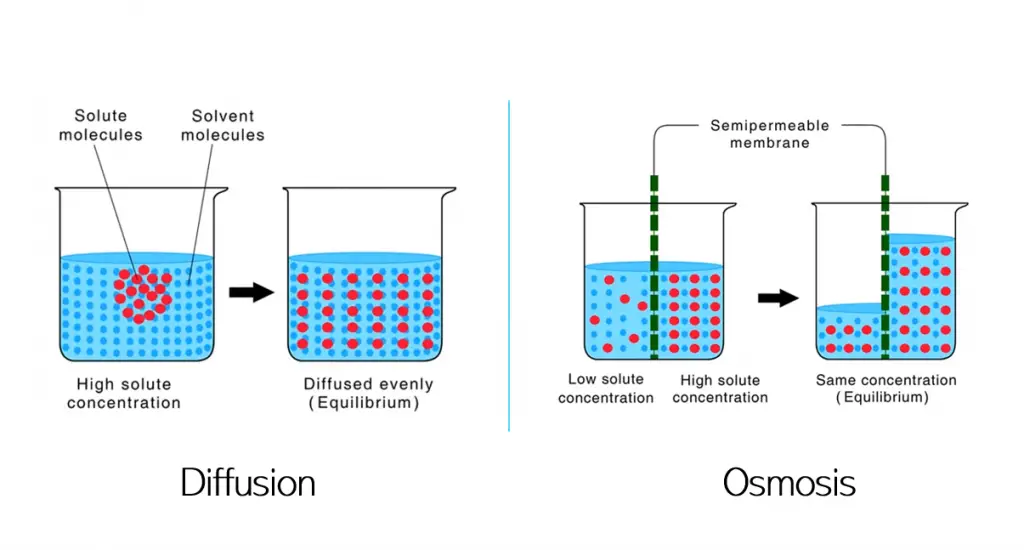
Diffusion and osmosis are two of the three types of passive transport processes, the third being facilitated diffusion. Passive transport is the movement of molecules or ions with no external energy being required to facilitate this movement.
Diffusion is the movement of solute molecules from a high concentration to low concentration while osmosis is the movement of solvent molecules from areas of low solute concentration to high solute concentration across a semipermeable membrane.
In the first graphic for diffusion above, we can see that the solute particles in the solvent are aggregated in a high concentration. After some time, the solute particles will move through the solution and spread out until equilibrium is reached – this is essentially what diffusion is.
In the second graphic for osmosis, we can see that there is a semipermeable membrane splitting the beaker into two parts – one with a low solute concentration and one with a high solute concentration. In order to have the solute molecules equally spaced out and be in a state of equilibrium, the solvent molecules move from low solute concentration to high solute concentration in order to dilute the higher concentration area.
So now that we have an idea of what osmosis is, let’s dive into what reverse osmosis is all about.
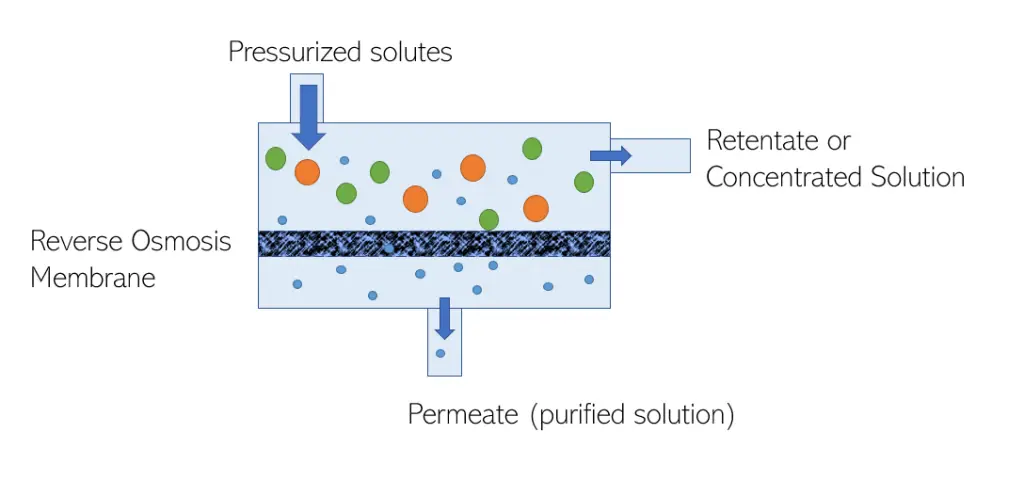
If osmosis is the movement of solvent molecules from areas of low solute concentration to areas of high solute concentration, then it makes sense that reverse osmosis is just the opposite.
The main principle behind reverse osmosis is to force a higher concentration solvent through a membrane in order to get a lower concentration solution free of certain impurities. Since you are forcing the solvent against its natural osmotic flow, you must have a pressure greater than the osmotic pressure in order to overcome this flow.
Check out this video describing how spiral wound reverse osmosis systems works
A major limitation to the reverse osmosis membrane is an increase in applied pressure is required for increases in osmotic pressure.
But why is this a limitation?
Lets look at the desalination of seawater as an example.
Seawater has high concentrations of salt dissolved in water, and so there will be a very high osmotic pressure pushing the water back to where it is coming from. In order to overcome this force, we need to increase the applied pressure and this significantly increases the overall costs of the reverse osmosis operation.
Chemicals like calcium sulfate or barium sulfate that collect on the reverse osmosis membrane surface can lead to irreversible fouling.
Due to the high concentration of the calcium or barium ions on the surface, the reverse dissolution reaction will be favored according to Le Chatelier’s principle. This will cause the calcium sulfate or barium sulfate to be precipitated out onto the membrane which will lead to scaling of the membrane.
Increasing the feed velocity flow over the membrane is critical in making sure that fouling is minimized. By increasing the turbulence of the flow, the contaminant build-up on the membrane surface is more likely to be swept away, thus decreasing the risk of membrane fouling and increasing membrane life span.
Particulates, ion concentration and free chlorine are the major components that can cause large operational challenges to reverse osmosis systems if they are not managed effectively.
For example, a small amount of chlorine can cause a catalytic reaction with the membrane and thus cause a leak.
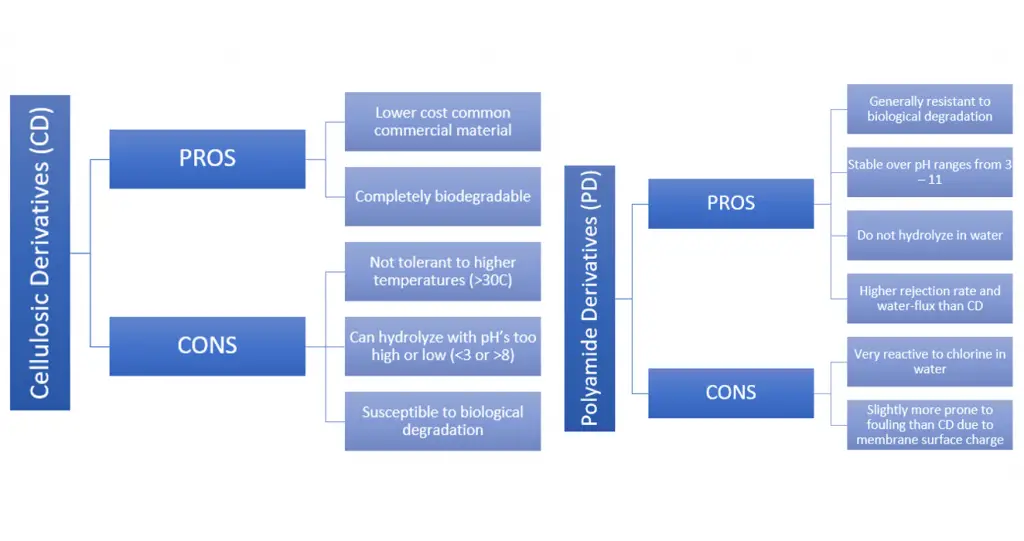
Two of the most widely utilized reverse osmosis membranes are made from polyamide derivatives or cellulosic derivatives. Generally speaking, the polyamide derivative based membranes are the better option in terms of water flux, rejection rate and stability. However, the cellulosic derivative membranes can be attractive if you are looking for a slightly cheaper and more environmentally friendly alternative.
The membrane configuration is the geometric shape Reverse osmosis membranes are typically fabricated as spiral-wound or hollow fiber.
The elements are designed with a cross flow configuration and have a membrane on either side of the feed water flow channel.
The most well known applications for reverse osmosis processes deal with the removal of salt from either seawater or brackish water. This process is commonly known as desalination and plays a large role in ensuring ultrapure water is produced for a variety of purposes such as human consumption or in the pharmaceutical or semiconductor industries where high purity water is required for sensitive equipment and materials.
In the midst of water shortages all around the globe, and with over 97 percent of the earth’s water locked up in undrinkable salt water, the reverse osmosis systems are a leading technology for many desalination applications.
Within the wastewater treatment industry, reverse osmosis systems play a critical role in making sure that high rejection rates are achieved to meet the purity specifications for the permeate stream. Wastewater comes from many different areas including municipalities, landfills and dumpsites, tanneries, electroplating industries and olive mills to name a few.
Depending on the final purpose for the water, the reverse osmosis process is often the back end of a much more complicated process. For example, typical municipal wastewater streams go through multiple levels of physical and chemical processes to remove suspended solids, particulates, and other harmful contaminants before being sent through a reverse osmosis module which acts as a final “polishing” step to allow for drinkable water.
Organic compounds and total dissolved solids are typically the major components removed by reverse osmosis in the wastewater application. Phenols, xylenol, and benzeneacetic acid are organics that cause high degrees of fouling which can cause a decay in reverse osmosis flux over time, and thus are removed in the process. Heavy metals and salts can also be removed depending on the final application of the water.
It is important to note that reverse osmosis as a pressure driven membrane separation process is just one of four major procedures that are conducted on waste water to ensure a safe transformation. The other three procedures are physical separation, chemical additions, and biological mechanisms.
Reverse osmosis is commonly utilized in the production of juices, dairy and non-alcoholic beverages to name a few.
Juice concentration is a major application for reverse osmosis membranes. Concentration can be done in a variety of ways, thermal based methods being one of the most common to heat off the water. However, there are disadvantages to this method including high energy consumption, high costs and the inability to utilize temperature sensitive solutions. Reverse osmosis solves these problems and also helps retain flavor and improve antioxidants which is very favorable in fruit juices.
WIthin the dairy industry, reverse osmosis is used in milk concentration, whey defatting, protein separation and recovery, and new dairy production creation.
Reverse osmosis plays an effective role as an alternative solution to dealcoholization using thermal based methods. Studies have shown that reverse osmosis membranes utilized to remove the ethanol from alcoholic beverages produce a non-alcoholic product with the quality and flavor fully preserved, all while reducing costs.
There are 2 things that make water considered “healthy”.
Reverse osmosis processes not only remove contaminants from the water, but also inhibit healthy minerals such as sodium, magnesium and calcium from passing through the membrane. The resulting water is an extremely pure form of water that is stripped of any contaminants or minerals.
So does this make the water healthy?
Yes and No.
Reverse osmosis water is still water, and essential for hydration, however, natural minerals in spring water still contribute a fraction of the minerals that human beings consume through their regular diets, and it is possible that eliminating these essential minerals from your diet can have an adverse impact on your overall health.
RO water is safe to drink. There are no negative effects to drinking this ultrapure form of water, however, it is important to keep in mind that you are eliminating a portion of your mineral consumption (100-200 mg/l of total dissolved solids) from your overall diet.
Bottled water can be water that undergoes reverse osmosis. Brands like Aquafina, Nestle Pure Life and Dasani all have processes that take their water through a rigorous multistage process where reverse osmosis is one step in the process. However, most of these manufacturers will include a remineralization stage to add the essential minerals back for a better taste and a healthier product.
Many major water bottle suppliers utilize reverse osmosis to remove all contaminants and minerals from their water, but then remineralize by adding minerals like magnesium, calcium and sodium.
You can add minerals to the water to make it more “healthy” as higher mineral water intake has been linked with decreased LDL cholesterol. Additionally, mineralization of RO water adds to the flavor profile of the water, with different minerals providing smoother, bitter or saltier tastes.
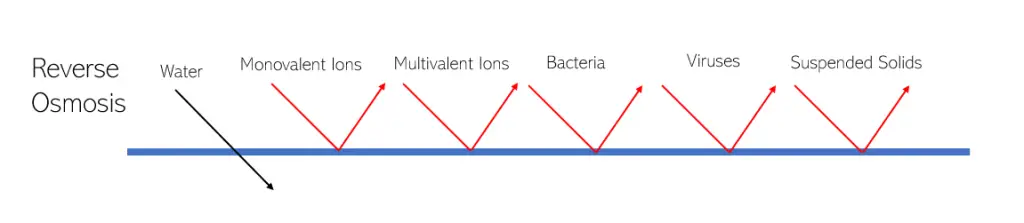
Reverse osmosis filters do filter out viruses. In fact, some viruses can even be removed with ultrafiltration membranes. Viruses have varying sizes and can be as small as 20nm, which would have a higher effectiveness in penetrating the membrane and entering the ultrafiltrate stream. In comparison, reverse osmosis have pore sizes that are less than 1nm.
Reverse osmosis does remove bacteria. The smallest bacteria are around 200-300 nm, while the reverse osmosis membrane has pores that are less than 1nm.
There are a couple conditions that need to be met in order for bacteria to grow and multiply – warm temperatures, moisture content, energy and nutrients, neutral pH levels and time are some factors that contribute to bacteria growth.
If you keep an open glass of reverse osmosis water out for a long enough period of time under the right conditions, microbes will grow and multiply. However, due to the lack of nutrients in the reverse osmosis water, it is much more difficult for bacteria to multiply as compared to regular tap water.
Remember, you have the information, but more importantly, you have the Engineer’s Perspective!
One Response
I find it fascinating that a reverse osmosis system makes it easier to remove contaminants from your drinking water. My uncle is interested in owning a countryside home so he can enjoy water activities around nearby water bodies. I should talk to him about finding a water works company that can install these systems around his home someday.