
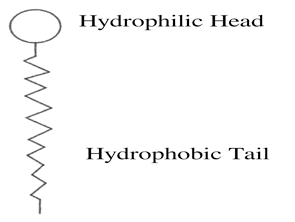
An industrial surfactant, or a ‘Surface Active Agent’ is a substance that reduces the surface tension in a liquid, and has commerical applications in industries such as chemicals, manufacturing and oil and gas.
As shown above, it contains a hydrophilic head and a hydrophobic tail.
So what is surface tension?
Surface tension is a property that occurs at a liquid’s surface which causes it to resist any outside force — this resistance is caused by the cohesive forces between liquid molecules.
Why reduce the surface tension of liquids, specifically water?
Reducing the surface tension of liquids serves many purposes depending on the intended industrial purpose.
For example, in the case of pesticides which are dissolved in water, reduction in surface tension means the force of attraction between water molecules is reduced, therefore the pesticide chemical is able to achieve greater coverage of the intended surface such as a farmers crop.
Another example is within packed distillation columns. A study by Anthony B. Ponter et al. demonstrated a “significant improvement” in efficiency of the packed distillation process through the use of a surfactant to increase the interfacial mass transfer area.
The hydrophobic tails, which are naturally attracted to soils (as we’ll discuss later), coil around them, and they’re pulled into the cleaning solution by the hydrophilic heads.
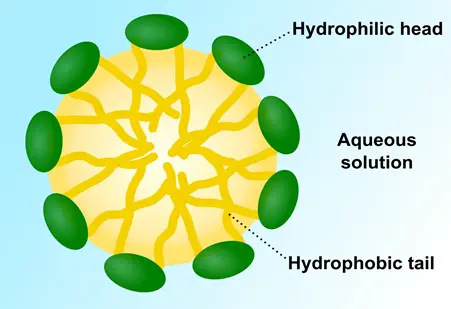
When a surfactant is added to a liquid (we will look at water in this example), some of the surfactant molecules move to the water surface.
Since the surfactant layer that has now migrated to the surface of the liquid exhibits weaker intermolecular forces between themselves, the surface tension of the overall mixture is reduced.
Some surfactant molecules that remain under the surface of the mixture aggregate in bundles to form micelles, further reducing the attraction between the water molecules.
Molecules from these substances occupy the spaces between the loosely attracted water molecules — this explains why there are substances that naturally don’t dissolve in water, but they do so when surfactants are added.
With time these micelles align such that their heads face water, while tails come together at the center, away from water.
Finally, a point is achieved where the surfactant molecules in most parts of the mixture do not form micelles. This point is called the CMC which stands for the Critical Micelle Concentration — the point at which an ideal amount of the surfactant is in the mixture.
Addition of the surfactant beyond this point doesn’t cause the surface tension of the liquid to reduce.
Surfactants are usually classified according to the charge on their hydrophilic head
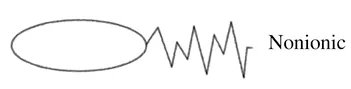
These are neutral surfactants which means that they have no charge on the hydrophilic end. Since they are neutral, they usually don’t react with ions such as calcium or magnesium that are typically found in hard water. Therefore, they are unlikely to form “soap scum” when used in hard water.
Examples of nonionic surfactants and their applications include:
Nonionic | Application (Used in) |
Sorbitan alkyl esters | Cleaners & polishes |
Polyoxyethylene glycol sorbitan alkyl ester | Ingredients for food |
Polyoxyethylene glycol alkylphenol ether | Spermicides |
Polyoxyethylene glycol octylphenol ether | Coatings |

Anionic industrial surfactants contain a negative charge (anionic functional group) at their head. This could be a phosphate, sulfate, sulfonate, or carboxylate group.
The negative charge enables the surfactant molecules to collect and suspend a wide range of positively charged particles like clay and soils — this explains why they are so popular in detergents and soil cleaners.
They can be created from a wide range of oils and fats such as palm, soybean, coconut, and tallow — this has led to the creation of milder surfactants, for example, Potassium Cocoate, which doesn’t cause skin irritation.
Anionic and nonionic surfactants are commonly used together to create multi-purpose detergents that can emulsify oily soils and remove particulate soils.
Examples of typical Anionic Surfactants and their uses include:
Anionic Surfactant | Application (Used in) |
Sodium stearate | Handsoap |
Dioctyl sodium sulfosuccinate | Toothpaste & coatings |
Lignosulfonate | Plasterboard & concrete plasticizer |
Perfluorooctanesulfonate | Skydrol |
Sodium lauryl ether sulfate | Bath products & shampoo |
Linear alkylbenzene sulfonates | Dishwasher & laundry detergents |
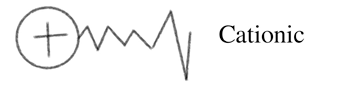
Cationic industrial surfactants contain a positive charge at the head. Most cationic surfactants are used in anti-fungus and antimicrobials in industrial and household cleaners.
They disrupt the cell membrane of these organisms, which results in their death. Due to their positive charge, they are highly attracted to the negatively-charged biomembranes of microorganisms.
In the end, their tails embed in the cell membranes of these microorganisms and cause proteins and lipids inside them to leak.
Nonionic and Cationic industrial surfactants are compatible. However, cationic and anionic surfactants can’t be used together — Due to the high electrostatic interactions between oppositely charged heads, the surfactants will fall from the mixture, reducing their effectiveness.
Anionic Surfactant | Application (Used in) |
Sodium stearate | Handsoap |
Dioctyl sodium sulfosuccinate | Toothpaste & coatings |
Lignosulfonate | Plasterboard & concrete plasticizer |
Perfluorooctanesulfonate | Skydrol |
Sodium lauryl ether sulfate | Bath products & shampoo |
Linear alkylbenzene sulfonates | Dishwasher & laundry detergents |

Amphoteric (Zwitterionic) industrial surfactants have both anionic and cationic properties on the hydrophilic end, which leads to the cancellation of the net charge.
Unlike nonionic surfactants, amphoteric industrial surfactants are always very sensitive to pH changes and will behave as cationic or anionic depending on the pH — acidic solutions create a positive charge while basic solutions create a negative charge.
The anionic part in amphoteric surfactants may be variable and have sulfonates. A good example is the CHAPS detergent (3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate).
Amphoteric Surfactant | Application (Use in) |
CHAPS detergent — (3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate) | UV/Vis spectroscopy, protein-protein binding |
Alkyl Amidopropyl Betaine | Laundry detergents, shampoo etc |
Alkyl Betaine | Hair & skin conditioners, cosmetics |
Industrial surfactants come with an HLB (Hydrophile-Lipophile Balance) value. The higher the number, the more water-soluble, and the lower the value, the more oil-soluble a surfactant is.
To get the best results, you should match the HLB requirement with the HLB value.
Natural surfactants (Biosurfactants) are biological compounds that have both ionic and cationic characteristics produced by bacterial activity on various substances such as waste material.
They’ve become popular due to their unique properties, e.g., low toxicity and ability to function under extreme conditions.
Yes and no. If the mixture had water and a surfactant only, it may irritate your skin and dry your hair. However, in most cases, co-surfactants, for example, fatty alcohols, are usually added in the (shampoo) mixture — this brings balance and eradicates the negative effects.
Cloud point temperature of polyoxyethylene-type nonionic surfactants and their mixtures.
(2003, February 15). ScienceDirect.
https://www.sciencedirect.com/science/article/abs/pii/S0021979702001625
Detergents and surfactants: a brief review. (2019, June 4). Open Access Journal of Science.
Retrieved March 1, 2022,
https://medcraveonline.com/OAJS/detergents-and-surfactants-a-brief-review.html
Synthetic and Bio-Derived Surfactants Versus Microbial Biosurfactants in the Cosmetic Industry: An Overview. (n.d.). MDPI.
Retrieved March 1, 2022,
https://www.mdpi.com/1422-0067/22/5/2371/htm
Remember, you have the information, but more importantly, you have the Engineer’s Perspective!