
Pressure Swing Distillation (PSD) is a separation process of pressure-sensitive azeotropes in industrial processes using two distillation columns operated in sequence at two different pressures.
An azeotrope is defined as a mixture of two or more liquids or molecules whose composition proportions cannot be changed by simple distillation. One of the more familiar azeotropic mixtures is the water/ethanol mixture (95.6:4.4 by weight) which will stay at this ratio regardless of how much you try to boil the mixture giving it a constant boiling point.
In PSD, the principle of separation is based on the fact that a simple change in pressure significantly alters the relative volatility of the mixture, which consequently shifts the composition of the azeotrope. As a result, the azeotropic point disappears from the system thus successfully separating the components in the mixture.
An azeotropic point is where the bubble point curve and dew point curve intersect in a temperature-composition diagram. When operating at constant pressure, the highest level of purity you can possibly achieve is at the azeotropic point, which cannot be changed without altering the distillation process. One of the techniques to break the azeotropic point is to change the pressure in the system. It is known that some binary azeotropic mixtures lose azeotropic behavior when system pressure is altered. In such a case, the separation of mixture components can be attained in the absence of an additional agent or entrainer (see Azeotropic Distillation).
For illustration purposes, let’s look at the x–y diagram for acetone–methanol at low pressure (1 atm) and high pressure (10 atm) below. Comparison between the two azeotropic points can be seen from the intersection of the equilibrium curves and diagonals shown in the figure, wherein the equilibrium curve shifted from the left side to the right side of the diagonal.
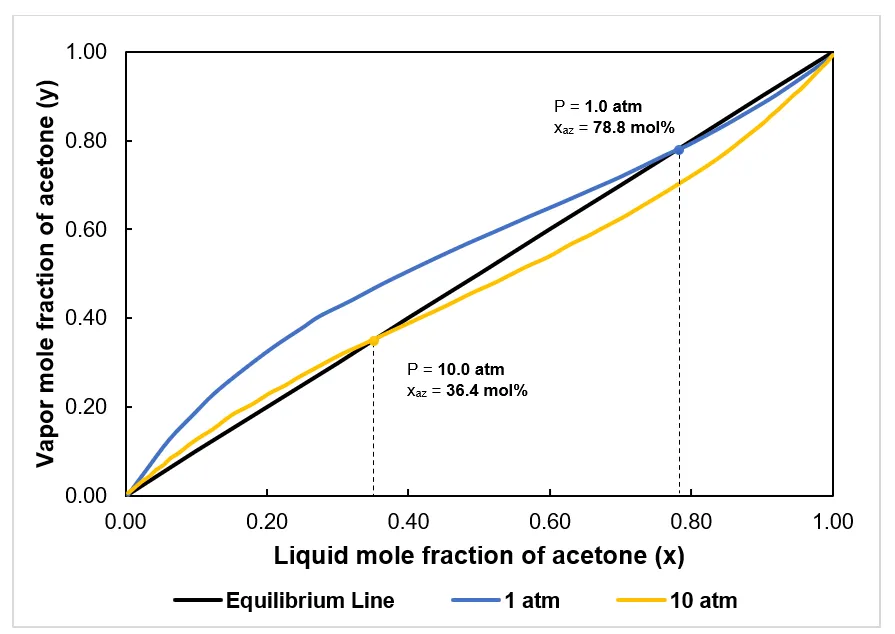
Here, we can see the azeotrope composition changes with the change in column pressure. For instance, at a pressure of 1.0 atm, the azeotropic composition of acetone is 78.8 mole%, while at 10.0 atm, the azeotropic composition of acetone is 36.4 mole%. This increase in pressure corresponds to a 53.8071% decrease in the value of the azeotropic composition.
This example implies that if the operating pressure is increased, the azeotropic point shifts to lower composition values of the light component. The significant positive change in the azeotropic point and enlargement of the relative volatility of azeotropic mixtures allow the separation to take place without any need for a separating agent. Moreover, such a large shift in the azeotropic composition of acetone indicates that a pressure-swing separation should be feasible for the acetone–methanol system.
Pressure-swing distillation applies to both minimum-boiling and maximum-boiling systems using a two-column system. The idea is to operate one column at low pressure and a second column at high pressure.
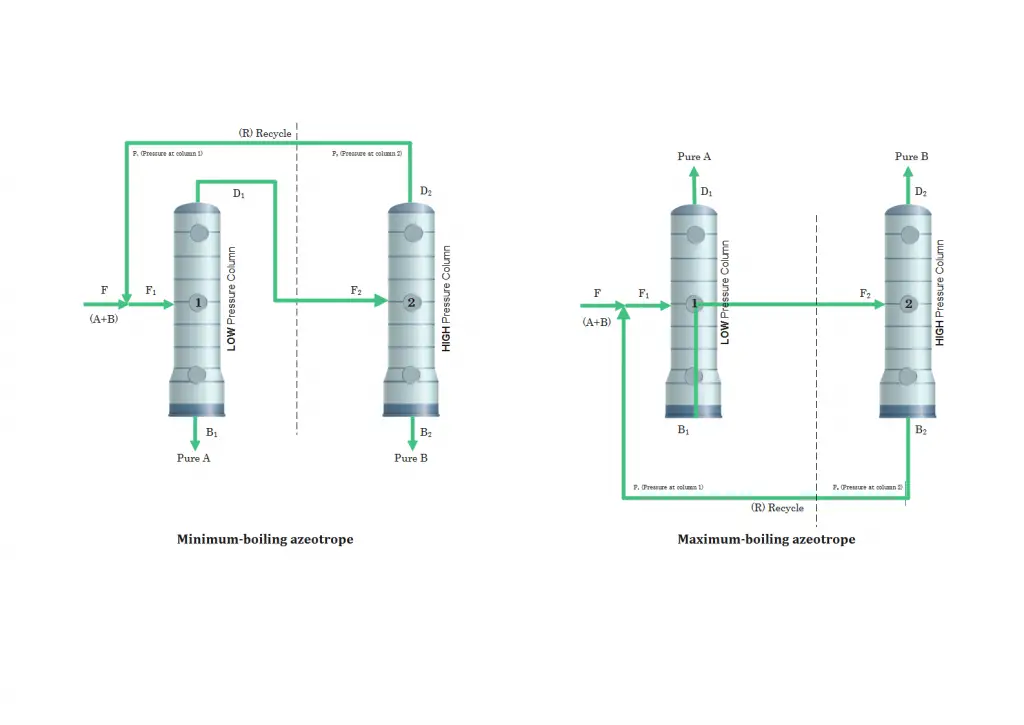
For a minimum-boiling azeotrope, the feed mixture (A+B) with R is fed to the low pressure column, in which the bottoms product is high purity A. The distillate that comes out of the first column, which has a composition at the azeotropic point at the low pressure is fed to the high-pressure column where a similar separation occurs, producing a bottoms that is high-purity B. The distillate, which has a composition close to the high-pressure azeotrope, is recycled back to the low-pressure column. For this type of azeotropes, the azeotropes (distillate streams) are recycled and the bottoms streams for both columns are the high-purity products.
For a maximum-boiling azeotrope, it has a similar process with the minimum-boiling azeotrope except that the azeotrope comes out of the bottom and the pure products at the top. The bottoms streams are recycled, not the distillate streams.
Azeotropic and extractive distillations are both methods of separating mixtures. They involve highly non-ideal phase equilibria and the addition of an agent to shift the phase equilibria of the components to favorable conditions. The main difference between the two is in the process of separating the mixture. In azeotropic distillation, the formation of an azeotrope is needed to separate the components of a mixture. In extractive distillation, no azeotrope formation occurs, and the added agent is essentially non-volatile.
A non-ideal binary solution showing a positive deviation is a minimum boiling azeotrope. For instance, a binary ethanol and water solution is considered minimum boiling azeotrope. On the other hand, a non-ideal binary solution showing a negative deviation is a maximum boiling azeotrope. For example, a binary nitric acid and water solution is considered maximum boiling azeotrope.
There are three modes of operation involving pressure swing distillation, namely, (1) batch operation, (2) semi-continuous operation, and (3) continuous operation. In batch operation, a single column operated at a certain pressure is recharged with a feed and emptied afterward. In semi-continuous operation, a single column operated at a certain pressure is also used; however, it is run continuously and periodically. Lastly, two columns are used in continuous operation at two different pressures.