
Distillation is a separation technique for solutions with components having a significant difference in boiling points — at least 20°C. There are different types of distillation, and they vary in application depending on the vapor-liquid behavior of the mixture to be separated. Fractional distillation, steam distillation, and vacuum distillation are some of its types.
But from here on out, we’ll be focusing on fractional distillation.
Fractional distillation separates miscible liquid solutions into their component fractions. It is the most suitable method when the difference in boiling points of components is by only a few degrees (less than 100 °C) in which it can be hard to separate the mixture in one run.
To complete the separation, there must be repeated cycles of evaporation and condensation. The basic principle for fractional distillation is that different liquids boil at different temperatures, as dictated by the vapor pressure properties of the components. When the mixture is heated, the more volatile component starts to boil first and vaporizes. It remains in a vapor state longer than the liquid component. This makes it possible to distill components of a complex mixture by fractions.
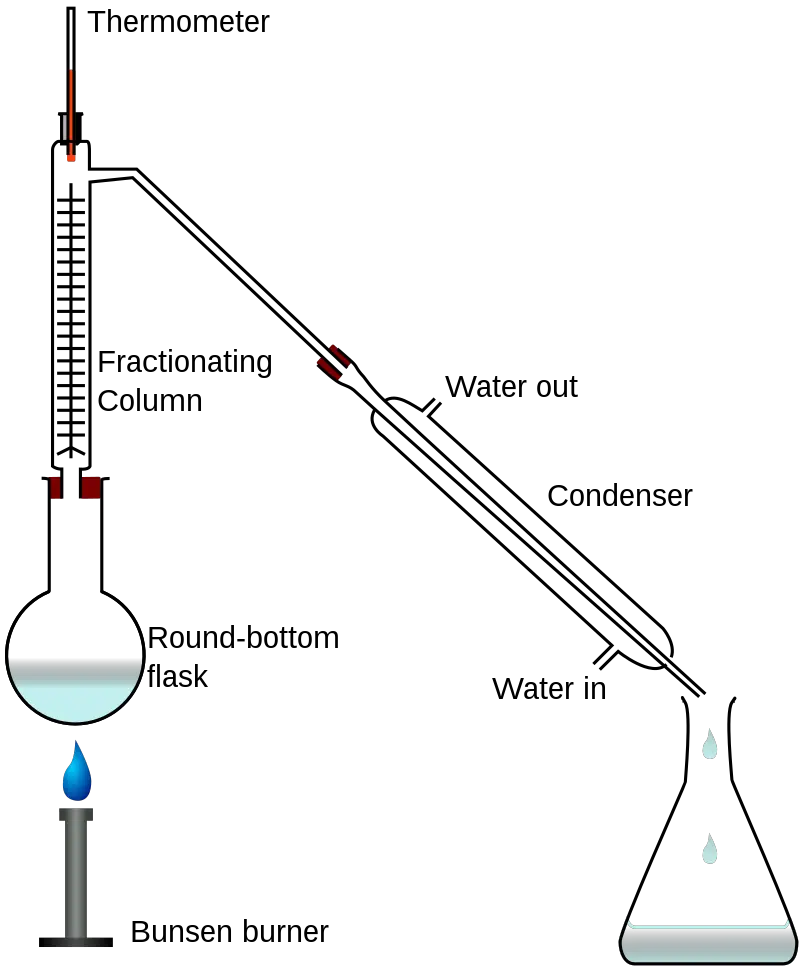
In the laboratory set-up above, the liquid mixture boils and the vapor travels up a glass tube called a “fractionating column”. This column, located between the flask containing the liquid mixture and the “Y” adaptor, improves the separation through the glass beads providing more surface area for vapor-liquid contact.
On an industrial scale, the distillation process occurs in packed or tray columns where packings and trays are used in place of the glass beads, respectively.
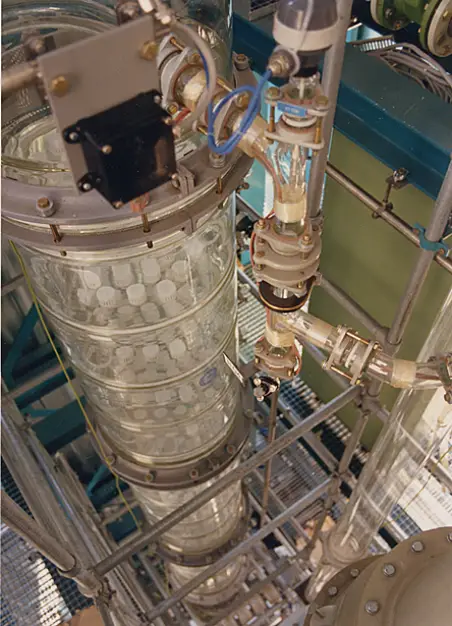
Both the glass beads (laboratory scale) and the tray or packed bed (industrial scale) serve as a “theoretical plate” on which the vapors can condense and then re-evaporate, and re-condense, resulting in distilling the liquid mixture many times over. Each theoretical plate corresponds to one vaporization-condensation cycle, which is equivalent to one simple distillation (see Simple Distillation vs. Fractional Distillation for more info).
The vapor eventually reaches the condenser where it cools and goes to the collector. Meanwhile, the less volatile component stays at the bottom, giving a better separation between the components of the liquid mixture.
Fractional distillation is commonly used in oil refineries. This process separates the various hydrocarbon components of crude oil to convert them into different petroleum products from the different distillates.
Industries also use fractional distillation for:
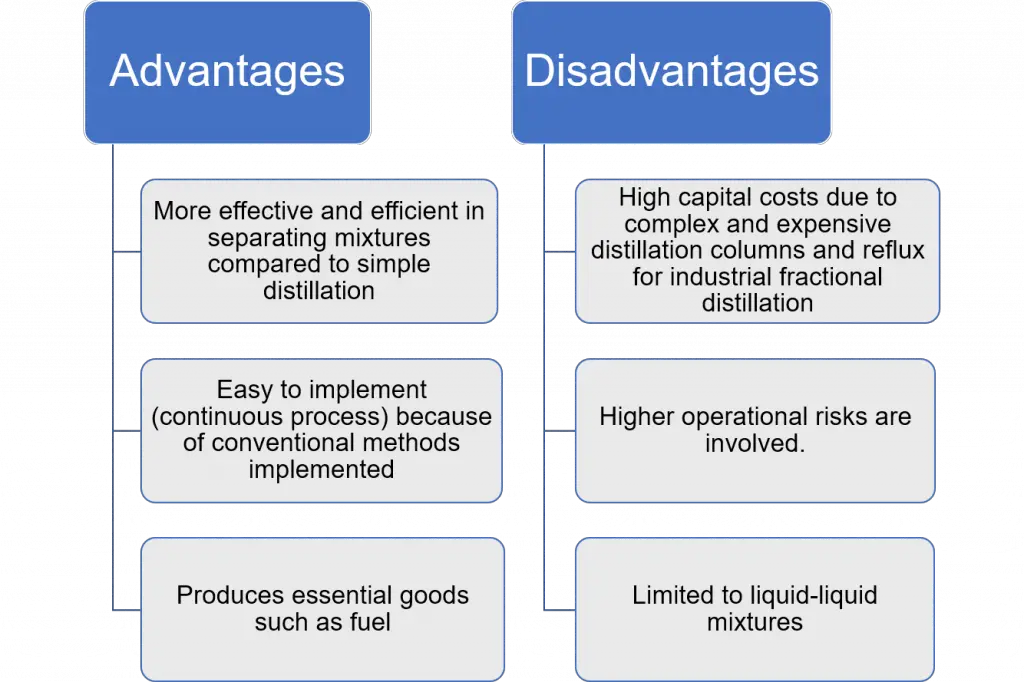
Fractional distillation is most efficient when separating complex mixtures into different component fractions with close boiling points. This is usually done in a continuous process requiring process control. Hence, it also has corresponding disadvantages such as:
In fractional distillation, theoretical plates are determined by the use of vapor-liquid equilibrium or VLE graphs and component balances (feed, distillate, and bottoms streams). The most common technique is the McCabe-Thiele method where every vaporization-condensation event corresponds to one theoretical plate.
Non-ideal solutions (azeotropes) cannot completely be separated by fractional distillation. Modifications or other separation methods (such as azeotropic distillation or pressure swing distillation) must be incorporated for a more effective separation.
Compared to simple distillation, fractional distillation gives better separation because of the glass beads/packings/trays in the fractionating column. These distillation column internals provide a better contact surface area on which liquid can repeatedly evaporate and condense.
One Response
It’s great that you elaborated that a proper separation would provide a balance for the materials to have a proper separation. The other night, my best buddy informed me he was looking for a glycol reclaiming solution that could provide a process without downtime concern for their industrial management. He asked if I had ideas on the best reclamation approach. I appreciate this valuable distillation guide article for the best planning approach. I’ll tell him it will be much better if they consult a trusted reclamation service as they can provide details about the process.